MAGEC Rod spinal implant lawsuits are something that parents in California and elsewhere are considering after studies’ results state there are several issues associated with the rods as well as complications stemming from their use. Among the findings were the following facts: revision surgery had to be undertaken, rods had failed to work or stopped working altogether, and children were diagnosed with metallosis and infection in connection to the rods. Parents may be able to receive compensation by seeking the help of a defective medical device attorney.
For more information, contact Attorney Group. We offer free, confidential, no obligation consultations. We can help answer your questions, and if you choose to pursue a case, we can connect you with an affiliated MAGEC Rod spinal implant lawsuit attorney who can assist you throughout the legal process.
What is the MAGEC Rod spinal implant?
The MAGEC Rod spinal implant is a fairly new medical device intended to treat early onset scoliosis in children through a method called fusionless surgery, during which a small rod is inserted into the child’s back along the spine. According to the Rady Children’s Hospital of San Diego, scoliosis is a condition that can be caused by poor muscle control of the spine, the “abnormal development of the bones in the spine,” or from a cause that doctors cannot determine. When scoliosis occurs, the spine will curve farther to the right or to the left than a normal spine, and in some cases, there is more than one unnatural curve. If left untreated, the abnormal curvature will continue to increase, causing problems for the child’s heart and lungs and affecting the child’s quality of life.
In some cases, a doctor may decide that the best treatment for the child is to perform the fusionless surgery and with traditional systems, the rods must be withdrawn and replaced with rods of a different length every six months. This is done to accommodate the child’s spinal growth and can extend for several years until the child reaches a stage where the spine has matured, at which time, the final set of rods will be removed. However, this can be difficult for children because each surgery requires a healing period and they are unable to engage in normal activities until the healing period ends. The MAGEC rods change this method with a technology that gives them the ability to automatically adjust within the child’s body for as long as needed, thereby eliminating the need for continuous surgeries.
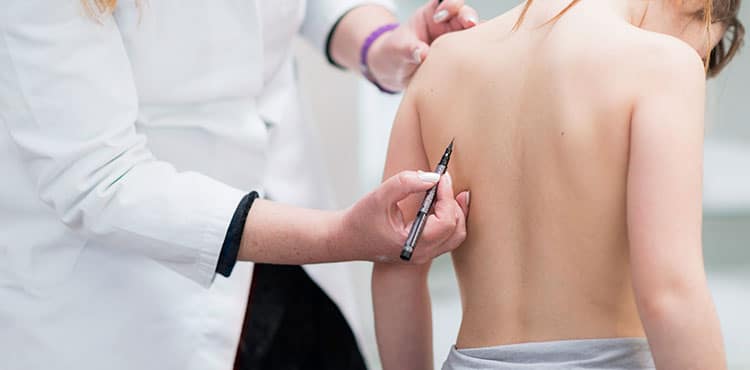
How does the MAGEC Rod spinal implant work?
The secret to the MAGEC rod is the insertion of a magnet, which is used as a control mechanism to turn the rods either left or right, which causes them to grow longer or become shorter. The magnet, which is programmed, is given its commands by an external remote control placed on the skin of the child’s back and operated by a doctor who has been trained in its use. The adjustment takes a short amount of time and there is no healing period, so the child can usually engage in physical activity, depending on their condition. NuVasive, the company that manufactures the rods, states that there are several benefits associated with their rods and these are: lower costs than other fusionless surgery methods, less psychological stress on the child, no need for repeated surgeries and the child is exposed to less anesthesia.
MAGEC Rod spinal implants and associated complications
When the MAGEC rod system received clearance from the U.S. Food and Drug Administration in 2014, it was marketed as a safer alternative for doctors to use on children with early onset scoliosis. However, studies that have been conducted on the children who received these rods, and on the rods themselves, have shown that the rods may not be as safe as first believed. Many children, it appears, have suffered from complications connected to the MAGEC system and even had to undergo unexpected surgery to have the rods removed.
Among the complications reported is the condition of metallosis, which is defined as a build-up of metal particles in the tissues surrounding a medical device, leading to symptoms that include infection; heart problems; hearing issues; and in some adult cases, death. In fact, when studies concentrated on the rods, it was discovered upon inspection that tiny particles of the titanium had accumulated within them, there was evidence of wearing inside the rods and on their surface, and that components were fractured.
MAGEC Rod spinal implant lawsuit news
October 2015 – A study in Orthopaedics & Traumatology: Study & Research claims that MAGEC rods are not as effective in treating early onset scoliosis than more traditional systems.
December 2016 – The European Spine Journal published a study conducted on nine MAGEC rods and researchers wrote that they found each rod contained some form of damage.
June 2017 – Bone & Joint Journal published a study that looked at children who received the MAGEC rod system and stated that 22 percent developed complications from the rods that required revision surgery to correct.
January 2018 – a United Kingdom study examined 34 rods after they were removed from children and stated that within each rod was an accumulation of titanium debris.
Is there a MAGEC Rod Spinal Implant Class Action?
There is no MAGEC Rod spinal implant class action pending as of March 2018. MAGEC Rod spinal implant lawsuit attorneys, however, are currently examining the findings of recent studies conducted on the rods and are meeting with concerned parents in California. Until these initial investigations are concluded, it is unknown if a class action will be certified for families whose children have suffered as a result of the adjustable rods. Instead, if multiple MAGEC Rod spinal implant lawsuits are filed against the manufacturer or others, alleging injuries and other damages caused by the MAGEC Rod spinal implant system, it is anticipated that these lawsuits will be consolidated for discovery and other pretrial proceedings.
When cases are consolidated in this way in federal court, it is called multidistrict litigation (MDL), and on a state level, it is known as a state court consolidated proceeding. MDLs are distinct from class actions, and it is generally agreed that consolidating cases instead of proceeding in a class action is a more efficient and effective way of handling claims arising from injuries caused by defective medical device products.
The Time You Have to Pursue a Claim is Limited. Contact Us Today.
For more information, contact Attorney Group for California. You can fill out the form on this page or contact us by phone or email.
After you contact us, an attorney will follow up to answer questions that you might have. There is no cost or obligation to speak with us, and any information you provide will be kept confidential.
Please note that the law limits the time you have to pursue a claim or file a lawsuit for an injury. If you think you have a case, you should not delay taking action.





