Metal-on-metal hip implants from several manufacturers have been recalled since 2008, leading to hip implant lawsuits in Iowa and throughout the country. Some of the implants recalled include, but are not limited to:
 Depuy ASR XL Acetabular System
Depuy ASR XL Acetabular System- Stryker Rejuvenate and ABG II
- Smith & Nephew R3 Metal Liners of the R3 Acetabular System
- Biomet M2a Magnum
- Wright Medical Technology Conserve and Profemur Z Stem
- Zimmer Durom Acetabular Component
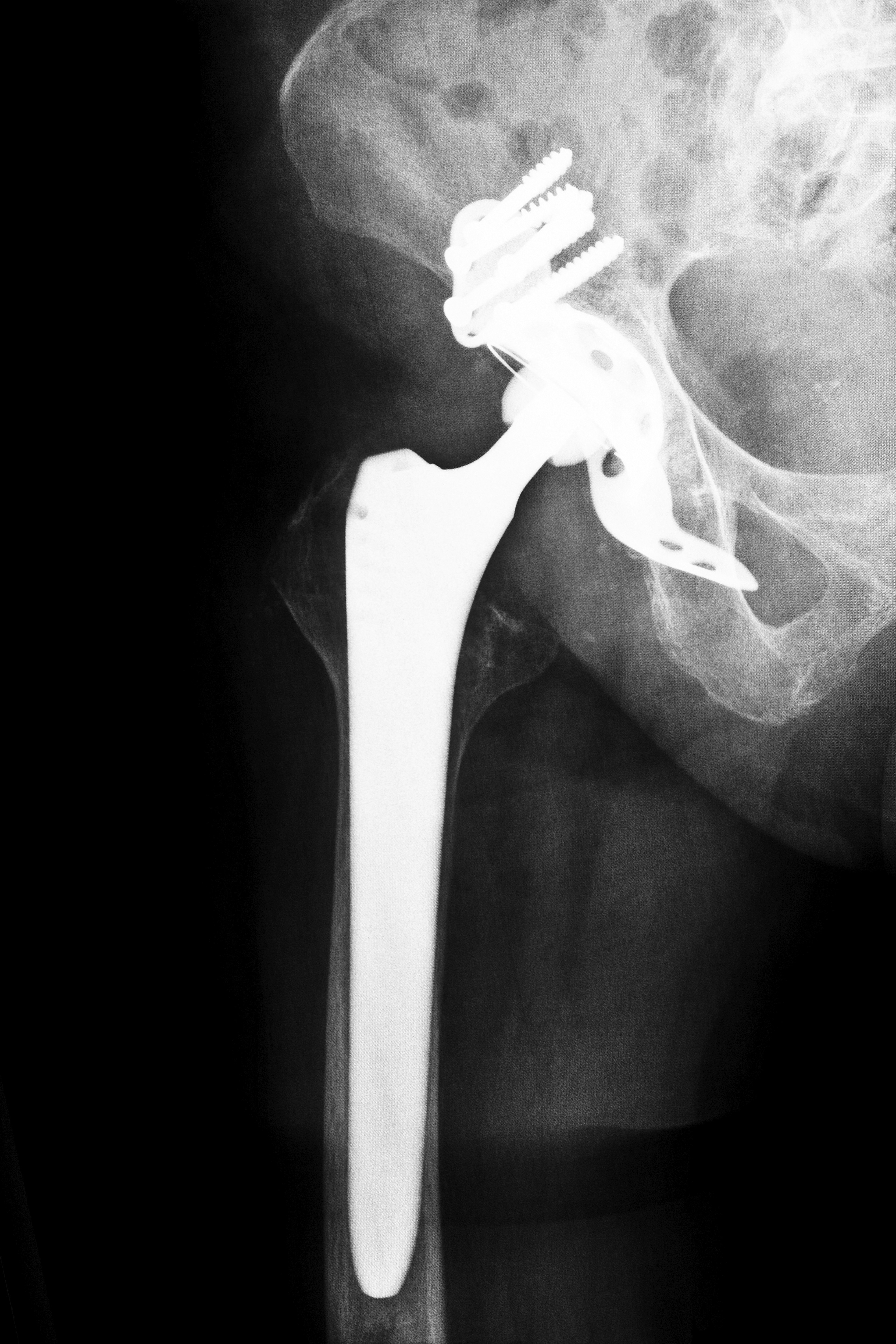
An Iowa woman filed one of the more than 3,000 hip implant lawsuits against DePuy, joining others who suffered significant injury due to complications from their Pinnacle Hip replacement system. Documents in the hip implant lawsuits claim that the Iowa woman had the system implanted in July 2005. Not long after the surgery, the woman began suffering from painful complications that included hip tenderness and difficulty walking. In January 2013, due to constant pain, she underwent risky revision surgery to remove and replace the device, which doctors discovered had failed. The Iowa woman claims that the revision surgery was far more painful than the initial surgery and that she is now at risk of chronic pain, bone loss along with other serious medical problems.
Many patients say that the manufacturers of the devices were aware of the problems, but concealed them from doctors and patients. The FDA says that metal-on-metal replacement hip systems have a metal ball that rotates within a metal cup. Through natural movement, the ball and cup rub against each other that can cause metal fragments to break off from the device, falling into the hip joint. The metal fragments can also enter the bloodstream, causing damage to other parts of the body. In one report, the metal fragments found were between one and two centimeters in size. In addition, reports began to surface in 2010 that the metal-on-metal implants were failing more quickly than those made of other substances. This has led to additional hip implant lawsuits in Iowa and other states as patients believe that the manufacturers were aware of the problems, yet kept them from the public.
When the metal fragments enter the bloodstream, there is a danger that they could travel to the brain. There is not a significant amount of research on how damaging metallosis, a condition where patients have higher than normal levels of certain types of metals in their bloodstream, can be to brain tissue and other organs. There is initial research that high metal levels in the bloodstream could lead to rare forms of cancer. The FDA recommends that any patient with a metal-on-metal hip implant have frequent blood tests to determine whether they have high metal levels in their blood even if they are not experiencing problems with their implant.
Considering Joining Hip Implant Lawsuits in Iowa?
If you or a loved one has suffered after the implantation of a hip replacement device, such as device failure, chronic pain or metal poisoning, or if a loved one has died of complications related to a hip replacement device, contact Attorney Group for Iowa to learn whether you qualify for a hip replacement lawsuit. You can reach us online or by telephone to set up your free initial consultation.