 MAGEC Rod spinal implant lawsuits are expected to be filed as more information comes to light regarding the safety concerns associated with the device. These concerns were raised in recent studies that show children have had to undergo revision surgery after suffering from complications directly associated with the implant. Complications have included the following: broken rods, metallosis, premature failure, failure to work at all, dislocation of the rods and infection. Parents may seek appropriate compensation on behalf of their children with the help of a defective medical device attorney.
MAGEC Rod spinal implant lawsuits are expected to be filed as more information comes to light regarding the safety concerns associated with the device. These concerns were raised in recent studies that show children have had to undergo revision surgery after suffering from complications directly associated with the implant. Complications have included the following: broken rods, metallosis, premature failure, failure to work at all, dislocation of the rods and infection. Parents may seek appropriate compensation on behalf of their children with the help of a defective medical device attorney.
For more information, contact Attorney Group. We offer free, confidential, no obligation consultations. We can help answer your questions, and if you choose to pursue a case, we can connect you with an affiliated MAGEC Rod spinal implant lawsuit attorney who can assist you throughout the legal process.
What is the MAGEC Rod spinal implant?
Every year, thousands of children are diagnosed with scoliosis, a condition that affects the growth of the spine. When a child is under the age of ten, they are diagnosed with early onset scoliosis and these children often face multiple health challenges such as cerebral palsy, spinal tumors and spina bifida. As these children grow older, they risk developing problems with their respiratory system and so doctors will often recommend fusionless surgery. Traditional surgery involves the use of a spinal rod, which is designed to keep the spine deformity from growing more severe. However, these rods must be replaced every six months in a surgical procedure with new rods that accommodate the growth of the spine through maturity.
In 2014, a company called NuVasive received approval from the U.S. Food and Drug Administration to launch a new spinal rod system called MAGEC and this system was implemented by The University of Iowa Children’s Hospital in January 2015. Unlike regular spinal rods, MAGEC rods are designed to adjust to the child’s body so there is no need for repetitive surgeries or for the healing periods these surgeries require. The decision to use MAGEC is made after the doctor examines several factors such as the child’s current health, if the child needs to have future MRI’s, the child’s previous health history, the child’s age and the child’s weight. If MAGEC is recommended, the rod is inserted through a small incision in the child’s back, and then anchored to the spine at the top and the bottom. When the child reaches maturity, the rod is then permanently removed in a surgical procedure.
How does the MAGEC Rod spinal implant work?
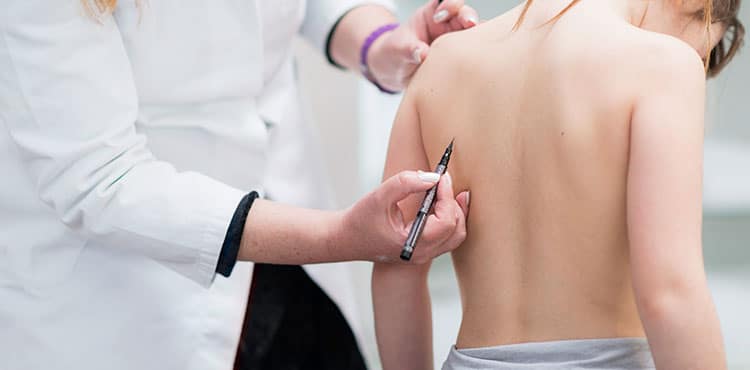
MAGEC uses magnet technology; a special magnet, that can be programmed, is put into the rod itself and the magnet controls the telescopic feature of the rod, which can be shortened or lengthened. A doctor, who is trained in how to make the adjustment, uses an external remote control to tell the magnet the direction to rotate. When the child comes in for the adjustment, the doctor will have the child lie on a table, their back facing upward, skin bare. The doctor puts the remote control on the child’s back, against the skin, and then operates the remote in a relatively painless procedure that takes a matter of minutes.
MAGEC Rod spinal implants and associated complications
When MAGEC was launched by NuVasive, no testing or studies were required for FDA approval because the rods are similar to those used in traditional fusionless surgery. However, researchers decided to conduct independent studies to evaluate the effectiveness of the rods used in the system, and these studies revealed several issues that have alarmed parents. Concerning the children who were given the MAGEC system, studies showed that a high number of children were required to undergo revision surgery due to complications with the device.
Complications that were reported include broken rods; rods that did not work or failed prematurely; and the development of metallosis – a condition caused by the accumulation of metal that has chipped off of a medical device and that can generate infection, problems with vision or hearing, and in some cases, heart problems. When one study dissected 34 rods that had been removed from children, researchers discovered metal debris within them as well as pieces of the rods that were fractured in some manner. In fact, all 34 rods had evidence of some form of decay on them.
MAGEC Rod spinal implant lawsuit news
October 2015 – Evidence is presented in a study on fusionless surgery published by Orthopaedics & Traumatology: Study & Research, that traditional spinal rods were actually more effective in their functioning then the MAGEC system.
December 2016 – Nine MAGEC rods were examined in a study published in the European Spine Journal and all were found to have some type of damage on them.
June 2017 – A study, printed in Bone & Joint Journal, states that 22 percent of children studied were required to go through a revision surgery that removed the MAGEC rods due to complications.
January 2018 – The newest study on 34 rods in the United Kingdom found titanium debris in all of them.
Is there a MAGEC Rod Spinal Implant Class Action?
There is no MAGEC Rod spinal implant class action pending as of March 2018. MAGEC Rod spinal implant lawsuit attorneys in Iowa, however, are currently meeting with parents who have learned of these new studies and are voicing concern. Until these initial investigations are concluded, it is unknown if a class action will be certified for families whose children have suffered as a result of the adjustable rods. Instead, if multiple MAGEC Rod spinal implant lawsuits are filed against the manufacturer or others, alleging injuries and other damages caused by the MAGEC Rod spinal implant system, it is anticipated that these lawsuits will be consolidated for discovery and other pretrial proceedings.
When cases are consolidated in this way in federal court, it is called multidistrict litigation (MDL), and on a state level, it is known as a state court consolidated proceeding. MDLs are distinct from class actions, and it is generally agreed that consolidating cases instead of proceeding in a class action is a more efficient and effective way of handling claims arising from injuries caused by defective medical device products.
The Time You Have to Pursue a Claim is Limited. Contact Us Today.
For more information, contact Attorney Group for Iowa. You can fill out the form on this page or contact us by phone or email.
After you contact us, an attorney will follow up to answer questions that you might have. There is no cost or obligation to speak with us, and any information you provide will be kept confidential.
Please note that the law limits the time you have to pursue a claim or file a lawsuit for an injury. If you think you have a case, you should not delay taking action.





