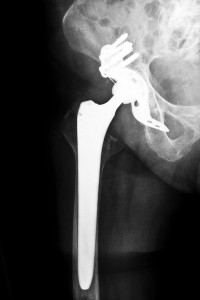
Stryker Orthopedics, manufacturer of the ABG II and Rejuvenate hip replacement systems, issued a recall of the two systems in July 2012 following increased reports of early device failure. In some cases, the ABG II devices failed as early as two to three years following implantation despite a design that was intended to last between 15 and 20 years.
Attorney Group for Iowa offers free consultations to those who have been injured by the ABG II hip stem and who may be considering filing a Stryker hip replacement lawsuit for compensation. To learn more about your options, contact us today. After we provide you with a free case evaluation, we can put you in contact with one of our affiliated attorneys who can file your Stryker hip replacement lawsuit on your behalf.
Early Device Failure, Other Complications Noted
The U.S. Food and Drug Administration (FDA) approved the ABG II Modular System in November 2009. The ABG II device is made with more components than other types of hip stems, including a metal neck, an acetabular cup, a femoral stem, and a ball. Because it was designed for younger, more active patients, the extra parts were intended to help to provide recipients with a greater range of motion.
Plaintiffs who are currently pursuing claims against the company allege that the additional parts merely meant an increased risk of problems and complications. One of the most commonly reported complications, early device failure, can result when friction causes the metal parts to release metal shards into the patients’ bloodstream. In its recall, Stryker acknowledge that these elevated metal levels could cause the patient to suffer from adverse local tissue reactions, swelling, and metallosis, also known as metal poisoning.
You may be eligible to file an Iowa Stryker hip replacement lawsuit if you suffered from:
- Early device failure
- Difficulty walking
- Trouble maintaining balance
- Pain
- Infections
- Swelling
- Tissue damage
- Temporary or permanent disability
Unfortunately, several victims of the ABG II who underwent revision or removal procedures reported that the subsequent surgeries were not always successful. As a result, patients may be confined to a wheelchair either temporarily or permanently due to pain and limited mobility. Individuals may consider filing an Iowa Stryker hip replacement lawsuit to seek compensation for these added medical expenses or for lost income if they were unable to return to work.
In December 2013, the first four Stryker hip replacement lawsuits were resolved for an undisclosed amount. Pending lawsuits accuse Stryker of negligence because it failed to adequately test the ABG II and Rejuvenate systems before releasing them. Plaintiffs also allege that Stryker neglected to warn physicians and consumers of the risks of side effects like metal poisoning and early device failure. According to Stryker, the combination of lawsuits and the recall could cost the manufacturer upwards of $1.2 billion.
Learn More about Filing an Iowa Stryker Hip Replacement Lawsuit
You may be eligible to file an Iowa Stryker hip replacement lawsuit if you received the Stryker ABG II hip stem and experienced complications caused by the device. Call Attorney Group for Iowa today. We can answer your questions, address your concerns, and review your case in a free, no obligation consultation to help you determine whether you have a claim. If you do, we can connect you with an affiliated ABG II attorney. He or she can assist you in filing your Iowa Stryker hip replacement lawsuit and help you seek compensation for medical expenses, lost wages, pain and suffering, and other damages to which you may be entitled.





