 A MAGEC Rod spinal implant lawsuit may be an option for parents in Pennsylvania whose children have been adversely affected after undergoing the surgical procedure. Parents are contacting attorneys after learning the results of studies conducted on the magnetic rods. These studies raise concern over the safety of the devices, reporting premature failure, metallosis, and failure of the device to lengthen. Parents of children who have suffered from complications may be able to file a lawsuit and seek appropriate compensation with the assistance of a defective medical device attorney.
A MAGEC Rod spinal implant lawsuit may be an option for parents in Pennsylvania whose children have been adversely affected after undergoing the surgical procedure. Parents are contacting attorneys after learning the results of studies conducted on the magnetic rods. These studies raise concern over the safety of the devices, reporting premature failure, metallosis, and failure of the device to lengthen. Parents of children who have suffered from complications may be able to file a lawsuit and seek appropriate compensation with the assistance of a defective medical device attorney.
For more information, contact Attorney Group. We offer free, confidential, no obligation consultations. We can help answer your questions, and if you choose to pursue a case, we can connect you with an affiliated MAGEC Rod spinal implant lawsuit attorney who can assist you throughout the legal process.
What is a MAGEC Rod spinal implant?
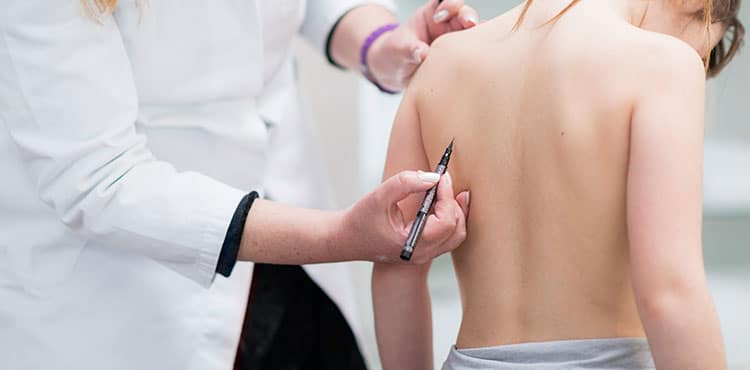
The MAGEC Rod spinal implant is a system designed to treat children with early onset scoliosis, according to the Children’s Hospital of Philadelphia. Traditional spinal rods must be replaced several times as the child grows. The MAGEC system eliminates the need for these invasive procedures by using a rod that can be adjusted with an external remote control. During the initial surgery, one or two rods are inserted into the child’s back along the side of the deformed spine. The rod(s) is attached with hooks, screws and other connecting hardware.
NuVasive, the company that manufactures the MAGEC rod system, says the system offers the following benefits to children and their families:
- Lower cost than traditional growing rods
- Eliminates the need for multiple hospitalizations
- Reduces the child’s exposure to anesthesia
- The child has fewer surgeries
Additionally, children who are treated with the magnetic rods will have a lower risk of suffering from the anxiety associated with undergoing multiple surgeries.
How does the MAGEC Rod spinal implant work?
When the rod(s) need to be adjusted, the doctor places a handheld remote control device over the child’s back and uses it to rotate the magnet. The rotation of the magnet causes the rod to shorten or lengthen, depending on what the doctor decides is needed. After the adjustment, or distraction, process, the child can resume normal activity in most cases since the procedure is relatively pain-free and can be done in a matter of minutes. The adjustable rod(s) provides support to the deformed spine, thus minimizing the impact of the early onset scoliosis. When the child reaches maturity, a surgical procedure is performed to remove the rod(s).
MAGEC Rod spinal implants and associated complications
Since the public launch of the MAGEC system in 2014, studies conducted in the U.S. and abroad showed several issues with the magnetic rod. In a two-year study involving 23 children, 14 problems were identified in 11 cases. Revision surgeries were performed on 10 children due to rod failure, breakage and metallosis – a condition where metal particles from the implant accumulate in the tissues.
A separate study on magnetic rods removed from patients revealed that the rods showed outward signs of wear and contained titanium wear debris, which could find their way into surrounding tissue.
Is there a MAGEC Rod Spinal Implant Class Action?
There is no MAGEC Rod spinal implant class action pending as of March 2018. Defective medical device lawsuit attorneys in Pennsylvania are still investigating the results of medical studies conducted and parents’ concerns. It is unknown if a class action will be certified for families whose children are adversely affected by the magnetic spine rod implants. Instead, if multiple MAGEC system lawsuits are filed against the manufacturer or others, alleging injuries and other damages caused by the MAGEC spinal rod system, it is anticipated that these lawsuits will be consolidated for discovery and other pretrial proceedings.
When cases are consolidated in this way in federal court, it is called multidistrict litigation (MDL), and on a state level, it is known as a state court consolidated proceeding. MDLs are distinct from class actions, and it is generally agreed that consolidating cases instead of proceeding in a class action is a more efficient and effective way of handling claims arising from injuries caused by defective medical device products.
MAGEC Rod spinal implant lawsuit news
October 2015 – A study printed in Orthopaedics & Traumatology: Study & Research, regarding fusionless surgery, states that an analysis of MAGEC rods showed they were less effective than expected.
December 2016 – A study in the European Spine Journal shows that all nine retrieved magnetic growth rods used for forensic analysis showed surface degradation on the telescopic rod and that some pins in the rods were fractured.
June 2017 – The Bone & Joint Journal publishes a study revealing that out of 195 juvenile patients, 22 percent required unplanned revision surgery due to complications caused by the MAGEC system.
January 2018 – A United Kingdom study examined 34 MAGEC rods that were removed from children and found signs of substantial damage inside the rods themselves as well as “titanium wear debris.”
The Time You Have to Pursue a Claim is Limited. Contact Us Today.
For more information, contact Attorney Group for Pennsylvania. You can fill out the form on this page or contact us by phone or email.
After you contact us, an attorney will follow up to answer questions that you might have. There is no cost or obligation to speak with us, and any information you provide will be kept confidential.
Please note that the law limits the time you have to pursue a claim or file a lawsuit for an injury. If you think you have a case, you should not delay taking action.





