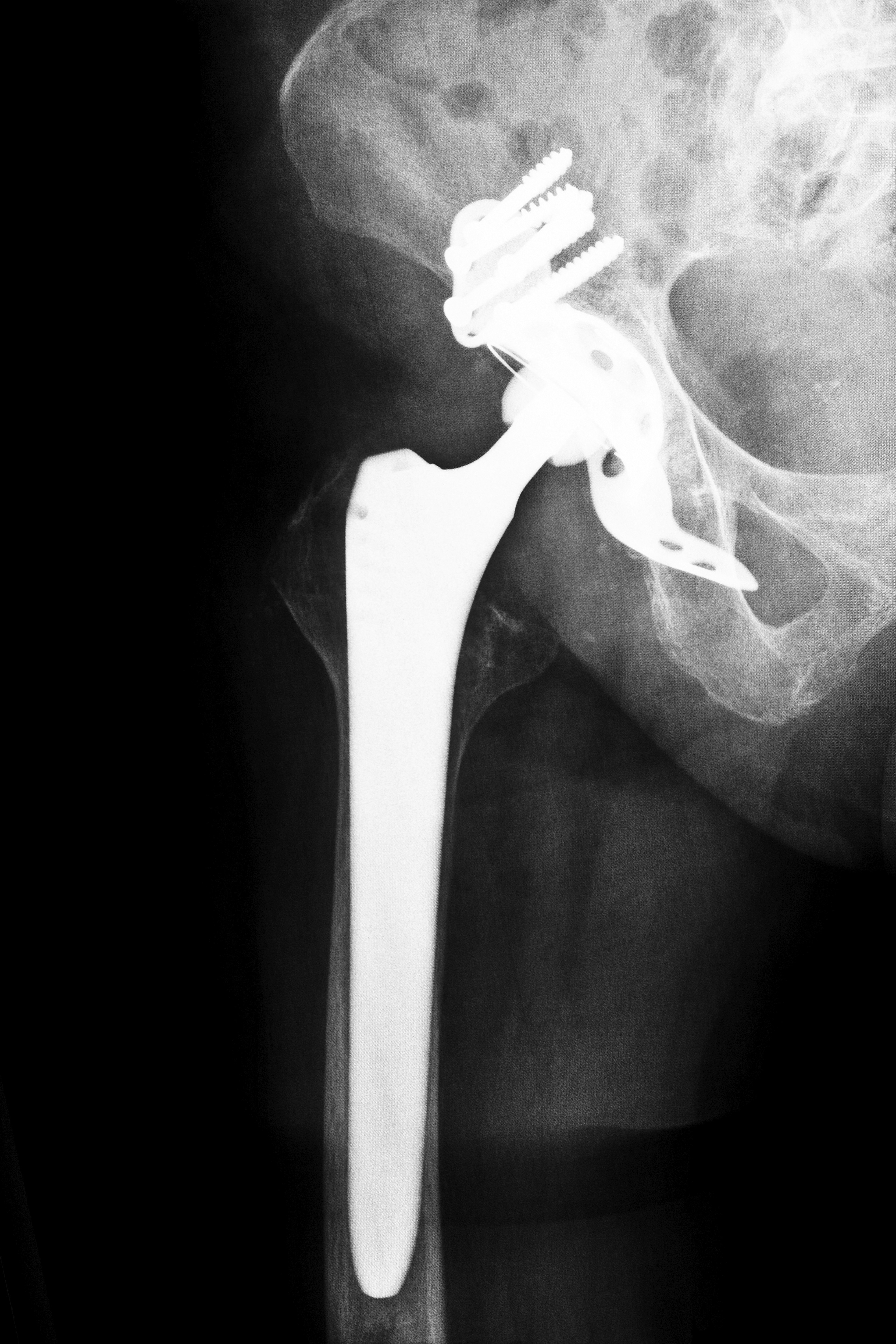
 In July of 2008, Zimmer, Inc. suspended all sales of its Zimmer Durom Hip Replacement Actabular Components, also known as the Durom Cup. The decision to do so was made because the cups had been shown to have a high rate of failure. In press releases regarding the suspension, Zimmer has placed the blame for Durom Cup failures on surgeons who implanted the device. They have claimed that faulty surgical technique was to blame. For their part, the surgeons have blamed the Durom Cup’s design. We are presently uncertain as to whether the company’s account is true, or whether the doctors are correct in their assertions regarding the quality of the Durom Cup. Arkansas Zimmer Durom Cup hip replacement attorneys can provide you with advice on whether to proceed with a lawsuit against the company. Attorney Group for Arkansas knows that you need enough data and solid legal advice to make an educated decision on seeking compensation.
In July of 2008, Zimmer, Inc. suspended all sales of its Zimmer Durom Hip Replacement Actabular Components, also known as the Durom Cup. The decision to do so was made because the cups had been shown to have a high rate of failure. In press releases regarding the suspension, Zimmer has placed the blame for Durom Cup failures on surgeons who implanted the device. They have claimed that faulty surgical technique was to blame. For their part, the surgeons have blamed the Durom Cup’s design. We are presently uncertain as to whether the company’s account is true, or whether the doctors are correct in their assertions regarding the quality of the Durom Cup. Arkansas Zimmer Durom Cup hip replacement attorneys can provide you with advice on whether to proceed with a lawsuit against the company. Attorney Group for Arkansas knows that you need enough data and solid legal advice to make an educated decision on seeking compensation.
The Durom Cup’s purpose was to be a reliable replacement product for patients who were younger and leading lifestyles that are more active. These patients would be living for longer periods of time than most hip replacement recipients after having the device implanted. The Durom Cup was designed to lower the risk of hip dislocations and to retain the hip-joint’s natural cavity.
The First Questions Raised
Arkansas Zimmer Durom Cup hip replacement attorneys will tell you that the first sign of trouble with the Durom Cup came via concerns voiced by one of Zimmer’s own consultants, Dr Larry Dorr. Dorr is an eminent orthopedic surgeon who stated concerns about the Durom Cup based on his own observation of its extremely high failure rate among his own patients. Dorr detailed the high failure rate in a letter to the American Association of Hip and Knee Surgeons in 2008. Of 165 patients who had undergone hip replacement surgeries involving the Durom Cup, 14 required revision surgery within two years of the initial hip replacement procedure. After the first set of revision surgeries, Dorr stopped using the Durom Cup and told the Food and Drug Administration about its high rate of failure.
Zimmer’s Response
- It launched an attack on Dorr, accusing him of lacking the technical ability to properly implant the Durom Cup.
- It conducted an investigation of the device, analyzing the manufacturing process.
- It retested the Durom Cup, verifying that its various elements met FDA requirements.
- Zimmer examined the records of production lots used in those surgeries where revisions were required.
- The company then looked at several clinical sites where the highest number of implants had taken place. They looked at thousands of patients. Zimmer then claimed to discover that at locations where surgeons had adhered to proper technique, the rate of revision surgeries was far lower than at those locations where that technique was not employed.
After completing its investigation, Zimmer suspended sales of the Durom Cup until a proper training program could be instituted for surgeons implanting the device. Sales of the device were suspended from July 2008 to August 2008. During this period, the device remained available in markets outside of the US.
Zimmer’s investigation uncovered no issues with the Durom Cup’s design, its manufacturing process or with the materials used to make it. Zimmer did acknowledge that due to the nature of the product’s design, it could be a technical challenge for a surgeon implanting it.
Indications of Durom Cup Failure
Patients saw no improvement in their condition after hip surgery. In some cases, their condition actually worsened as a result of the hip implant. This was so even though X-rays indicated no change in the Durom Cup’s position and doctors could detect no evidence that the device had loosened.
Patients who have suffered any of the following as a result of Durom Cup hip replacement surgery are entitled to seek compensation via one of Attorney Group for Arkansas’s affiliated Arkansas Zimmer Durom Cup hip replacement attorneys:
- Reduced quality of life
- Pain and suffering
- Lost wages
- Debt due to medical costs
Between the point where it was offered as a solution for patients and the suspension of sales, it is estimated that roughly 12,000 people had the Durom Cup implanted. The exact number of those patients who faced problems because of Zimmer’s failure to provide adequate training for surgeons is not known. If you have had the Durom Cup device implanted and have developed problems because of it, consulting with Arkansas Zimmer Durom Cup hip replacement attorneys may aid you in getting compensation. Contact Attorney Group for Arkansas today for a free consultation.