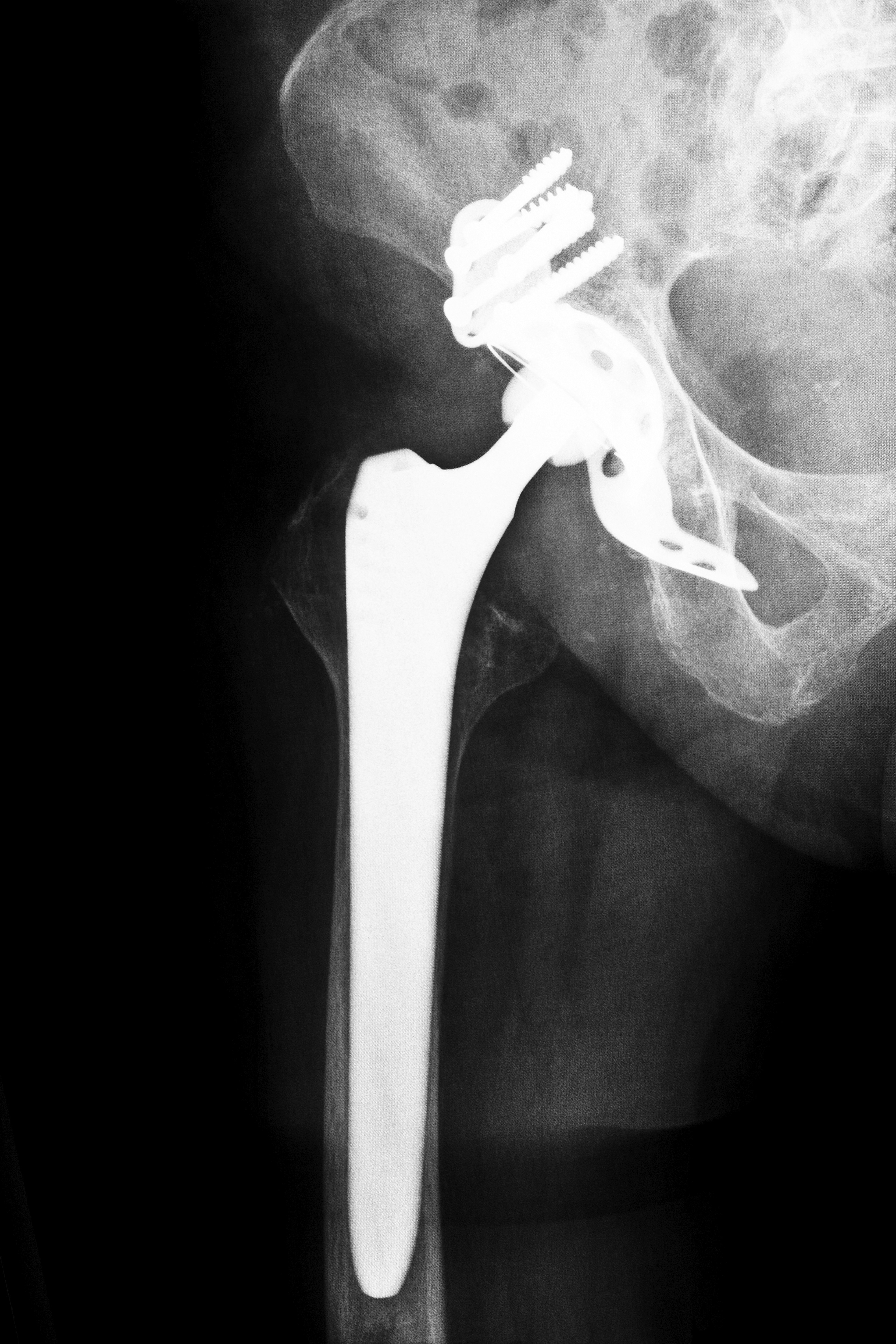
What is the Stryker ABG II Hip Replacement System and why should I look into a Arkansas Stryker Hip Replacement Lawsuits?
Stryker Corporation is the global leader in production and manufacturing of implant systems. In 2009, Stryker released the Stryker ABG II hip replacement model. The Stryker ABG II comprised plastic-on-ceramic components as well as metal and included a modular design to maximize mobility. The modular neck stems, in particular, was a significant innovation for several reasons. First, medical professionals could adapt the hip replacement device to the particular anatomy and needs of the patient’s body. Second, such attention to the patient’s biometrics meant that the device, in theory, could provide greater ease of use and mobility.
However, after three years on the market, Stryker ABG II hip replacement devices have proven to be high-risk medical products. Fretting, in which the metal component rub against each other causing dangerous wear-and-tear, was a key side effect. Stryker Orthopedics, as the largest manufacturer of hip replacement devices in the world, decided to issue a recall for the Stryker ABG II hip replacement device, which had been planted in thousands of patients globally in the previous three years. This recall came on the heels of multiple statements focusing on safety issues that were issued on Stryker’s website. While the orthopedic conglomerate admitted that the Stryker ABG II hip replacement device posed unreasonable risk to consumers, citing excessive instances in which hip revision surgery followed implantation, the company added a critical caveat, noting that the instance of such complications is ultimately low.
Since this voluntary recall, the FDA has issued recall for 21 metal-on-metal hip replacement devices. Stryker has taken steps to ameliorate conditions for individuals adversely affected by the Stryker ABG II hip replacement by establishing a 24-hour toll free hotline to voice complaints and encouraging individuals who have complications to contact medical professionals immediately. In addition, Stryker has taken the bold step of encouraging individuals who have not yet experienced any complications with their hip replacement device to investigate and discuss a post-operative procedure with their physicians as a cautionary measure. Stryker Orthopedics took measures to inform the medical and biomedical engineering communities of difficulties with the Stryker ABG II hip replacement. After assessing complaints, data, and safety risks associated with the device, Stryker ABG II produced a manual entitled “Urgent Field Safety Notice.” This manual detailed the hazards of fretting and corrosion, two side effects that implicate the metal-on-metal components of the device, and encourage physicians to seriously weigh the risks and benefits for each case.
What Are the Risks Associated with the System?
The Stryker ABG II hip replacement comprises several metal components made of cobalt and chromium. When fretting occurs, the structural integrity of the Stryker ABG II hip replacement device is compromised and particulate matter from the fretting enters the bloodsteam. Individuals whose devices have experienced fretting demonstrate higher chromium and cobalt ions in their bloodstream and, therefore, suffer from metal toxicity. Stryker hip replacement lawsuits, which represent the claims of thousands of individuals, have found the following complications post-surgery to be common:
- Fatigue
- Decreased mobility
- Metal toxicity
- Tissue and bone necrosis, or death
- Irritation and rashes around tissue
- Blood vessel damage
- Kidney and liver damage due to metal toxicity
- Bone fracture
- Bone loss
- Need for revision surgery
- Compromised lymph nodes, spleen, kidneys, and liver
- Neurological problems due to metal exposure
- Emotional and mental health issues
- Chronic, excessive, or unmanageable pain
- Hip immobility
- Heart problems
Attorney Group for Arkansas
If you or someone you know received a Stryker ABG II Hip Replacement System and have experienced complications after the implantation of the device, you may be eligible for compensation. Attorney Group for Arkansas comprises an extraordinary group of trial lawyers with decades of experiencing litigating such cases. Contact Attorney Group for Arkansas for a confidential, free, and thorough review of your case. Our attorneys understand the emotional stress that entering a Arkansas Stryker Hip Replacement Lawsuits with such complex litigation has for clients. We approach each case with great care and individualized attention and understand that each client has unique circumstances in his or her case. Our Stryker hip replacement lawsuits are seeking compensation for our clients for pain, suffering, medical expenses, loss of wages, employment-related costs, home-related costs due to surgery, emotional pain, among other issues. If you feel you or someone you know may be eligible to participate in Stryker hip replacement lawsuits, contact Attorney Group for Arkansas as soon as you are able. Certain statutes may limit opportunity to pursue litigation and receive subsequent compensation so expediency is key for all cases.