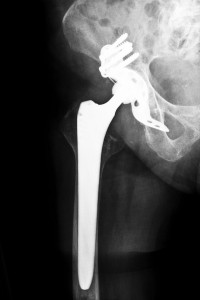
The Zimmer Durom Cup is an artificial hip device that has been used in hip replacement surgeries since 2006 in the United States. It differs from other hip replacement devices in that it is made of a single piece of material. However, Dr. Larry Dorr, an orthopedic surgeon from California, reported to the American Association of Hip and Knee Surgeons in April 2008 that some of his patients who had been implanted with the Zimmer Durom Cup implant were experiencing problems. His patients had told him that they were suffering from pain in their hip region a good three months following surgery. They also felt that the hip implant was loosening. They eventually required revision surgeries to correct the complications and Dr. Dorr felt that the amount of patients needing an additional surgery to fix the problems associated with the implant were greater than expected. Arkansas Zimmer Durom Cup Hip Recall Attorneys are brining liabilty lawsuits against the manufacturer
Zimmer Holdings, Inc. dismissed the reports initially that they were getting from doctors regarding problems with the implant. However, Zimmer conducted an investigation in May 2008 concerning the Durom Cup problems. Over 3,100 cases were thoroughly reviewed and it was basically concluded by Zimmer that the average orthopedic surgeon did not have enough training in the use of this device to properly handle the surgery. The company believed that their implant’s design along with the technology associated with it required a high degree of skill and precision during a hip implant surgery that involved the use of their device. They believed that this special technique was not a normal part of doctors’ procedures during a hip replacement surgery. Zimmer concluded that orthopedic surgeons needed very special training and specific instructions before they operated with this device.
Zimmer then suspended sales of the Durom Cup device in July 2008 in the United States, but they did not recall the implant because their investigation did not find any evidence of design or manufacturing defects of their product. Instead, the company plans to develop a specialized training program including detailed instructions regarding the specific technique surgeons need to use to properly implant this device to avoid problems and then reintroduce it back into the market.
Arkansas Zimmer Durom Cup Hip Recall Attorneys
Because Zimmer Holdings, Inc. neglected to properly provide warnings that special instructions and training were needed prior to implanting their product, many patients have suffered complications with the Zimmer Durom Cup. If you or someone you care about has experienced complications after receiving the Zimmer Durom Cup implant or has even had to undergo additional revision surgery to fix the problem, contact Arkansas Attorney Group so our member Arkansas Zimmer Durom Cup Hip Recall Attorneys can help determine if you could be eligible for financial compensation.





