If your child has been treated for early onset scoliosis with the MAGEC (Magnetic Expansion Control) system and suffered complications, including broken rods, implant failure, necrosis, metallosis, device failure or infection, contact Attorney Group for more information about your options.
We can answer your questions in a free and confidential consultation. If you wish to pursue a claim, we can connect you with an affiliated attorney who can file a MAGEC Rod spinal implant lawsuit on your behalf.
Studies conducted on the MAGEC Rod spinal system after its public launch show that up to 22 percent of children in one group underwent surgery to remove the rods due to complications and that the rods themselves showed multiple problems with corrosion, wear and tear, and failure to work properly.
After the release of several studies’ findings, MAGEC Rod spinal implant lawsuits may be pending. These studies reveal that since the system’s launch in 2014, problems have been attributed to the titanium rods implanted in young children. These problems include failure to operate as designed, premature failure of the rods, metallosis developing in children, broken rods and the presence of titanium debris within the rods. Parents may take legal action and seek compensation for the sufferings their children have experienced as a result of the MAGEC spinal implant rod.
For more information, contact Attorney Group. We offer free, confidential, no obligation consultations. We can help answer your questions, and if you choose to pursue a case, we can connect you with an affiliated MAGEC Rod spinal implant lawsuit attorney who can assist you throughout the legal process.
Have you seen a MAGEC Rod spinal implant lawsuit commercial?
You may have seen a MAGEC Rod spinal implant lawsuit commercial on television and wondered whether your child has been affected by the MAGEC Rod spinal implant they received, and if so, whether you are eligible to pursue a claim against the manufacturer or others. The purpose of this article is to provide you with additional information so that you have a better understanding of your options.
What is the MAGEC Rod spinal implant?
Sometimes, a child’s spine is curved more than it should be and when this happens, the diagnosis of early onset scoliosis is the result. To prevent the spine from developing into a more serious deformity, the doctor may decide that the child needs fusionless surgery. Normal fusionless surgery involves the insertion of rods, which are then replaced every six months throughout the child’s growth period, but in 2014, a new system called MAGEC, was released to the medical community, offering a new alternative to repetitive surgery.
MAGEC uses magnet technology that allows the rods to adjust, eliminating the need for semi-annual surgeries and the healing period that follows them. In the initial surgical procedure, the surgeon makes small incisions and then inserts one or two rods along the child’s spine, attaching them at the top and the bottom, similar to the procedure used for traditional spine rods. When the rods are no longer needed, they are removed in a second surgery. The new system saves money for families in the long term as well as lowers the emotional trauma the child will experience. Before a doctor decides to use the MAGEC system, however, several factors are considered such as the need for additional MRIs, the child’s age, health conditions the child currently has and the child’s medical history.
How does the MAGEC Rod spinal implant work?
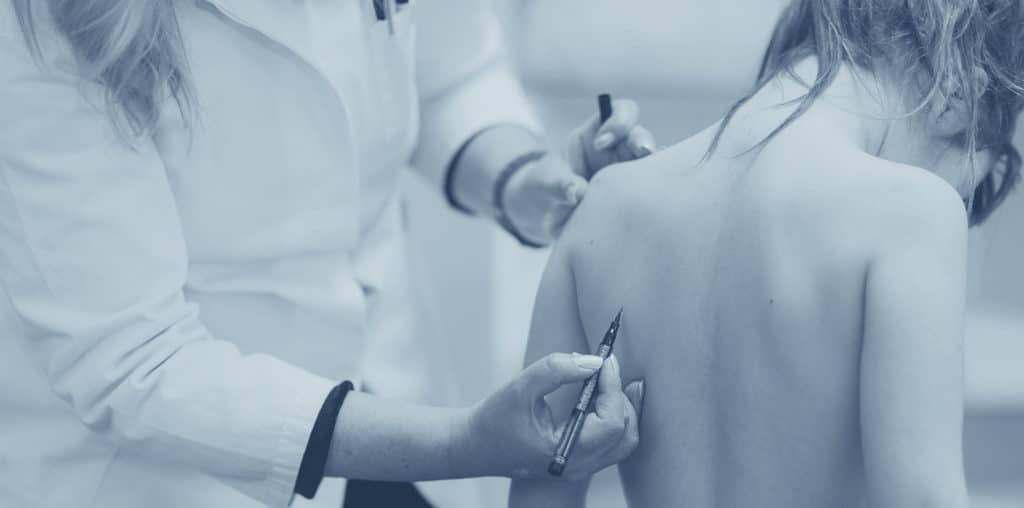
MAGEC rods are designed in the same manner as a telescope, enabling them to lengthen or shorten as needed. Inside each rod, a magnet is placed and the magnet controls the rotation of the rod mechanism through the use of an external remote control. The remote control is placed against the child’s bare back by a doctor who is trained in how to operate it. As the child lays, chest down, on a table, the doctor finds the position of the magnet and then uses the remote control to tell it which way to turn. The magnet then turns, activating the motorized rod.
MAGEC Rod spinal implants and associated complications
Due to the fact that the MAGEC system is similar to spine rods already in use on children, no studies were conducted prior to its clearance as a medical device by the U.S. Food and Drug Administration. However, after the device was implanted into several young children, both in the U.S. and abroad, studies were held to examine the effectiveness of the rods and the conditions of the rods themselves after they were removed from children in revision surgeries.
The results of these studies reveal complications experienced by children after implantation as well as condition issues with the rods after use. Complications include metallosis, or the accumulation of metal debris in surrounding tissues; infection; the need for revision surgery; rods breaking or dislocating while still implanted; and discoloration and pigmentation of surrounding tissues. When rods have been examined after usage, researchers found components that were fractured, signs of corrosion and surface wear. Studies also state that in some cases, the rods failed to work at all.
MAGEC Rod spinal implant lawsuit news
October 2015 – A study on fusionless surgery in Orthopedics & Traumatology: Study & Research, states that the MAGEC rods did not function as effectively as other rod systems on the market.
December 2016 – A study published in the European Spine Journal reports that the nine MAGEC rods examined all showed some form of damage after usage.
June 2017 – Bone & Joint Journal publishes a study conducted on 195 children who received MAGEC rods. The results revealed that 43 children, or 22 percent, suffered complications from the rods that required “unplanned revision surgery.”
January 2018 – A United Kingdom study noted “titanium wear debris” within all 34 rods that were examined.
Is there a MAGEC Rod Spinal Implant Class Action?
There is no MAGEC Rod spinal implant class action pending as of March 2018. MAGEC Rod spinal implant lawsuit attorneys are meeting with concerned parents and conducting investigations on the medical devices, but it is unknown if a class action will be certified for families whose children have suffered as a result of the adjustable rods. Instead, if multiple MAGEC Rod spinal implant lawsuits are filed against the manufacturer or others, alleging injuries and other damages caused by the MAGEC Rod spinal implant system, it is anticipated that these lawsuits will be consolidated for discovery and other pretrial proceedings.
When cases are consolidated in this way in federal court, it is called multidistrict litigation (MDL), and on a state level, it is known as a state court consolidated proceeding. MDLs are distinct from class actions, and it is generally agreed that consolidating cases instead of proceeding in a class action is a more efficient and effective way of handling claims arising from injuries caused by defective medical device products.





