 The DePuy ASR XL Acetabular hip replacement represented the latest in medical technology when it was approved for use by the U.S. Food and Drug Administration (FDA) in 2005. However, some of those who had received the device soon began to suffer from complications, leading to the need in some cases for additional surgery and, in turn, to legal action against the manufacturer. Residents of Mississippi who have received the ASR and have a result experienced health problems should be aware of their right to seek compensatory damages. The best person to help them consider their legal options is a Mississippi personal injury attorney.
The DePuy ASR XL Acetabular hip replacement represented the latest in medical technology when it was approved for use by the U.S. Food and Drug Administration (FDA) in 2005. However, some of those who had received the device soon began to suffer from complications, leading to the need in some cases for additional surgery and, in turn, to legal action against the manufacturer. Residents of Mississippi who have received the ASR and have a result experienced health problems should be aware of their right to seek compensatory damages. The best person to help them consider their legal options is a Mississippi personal injury attorney.
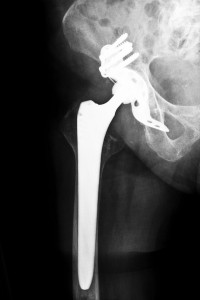
The all-metal design of the ASR was supposed to guarantee improved durability over previous implants. However, the design may have actually been a factor in the many failures that have been reported. In some cases, metal debris produced during the course of normal wear apparently led to aseptic lymphocyte-dominated vasculitis-associated lesions. Metal particles coming from the device may have also resulted in pseudotumors, which resemble actual tumors by the way they create swelling in human tissue. The FDA has in fact received more than 300 reports of health problems related to the ASR.
Initial research indicated that with a failure rate of more than 10 percent, about one in eight recipients of the ASR would require replacement of the implant. However, a subsequent study pointed to a much higher rate of failure, with around 20 percent of the ASR recipients requiring replacement surgery no more than four years after they had received the device and nearly 50 percent of them needing surgery no more than six years after implantation. Other patients who did not requiry surgery could have experienced symptoms related to the implant, including pain. Surgical procedures can be particularly expensive, and when such treatment is needed to counter the effects of a defective implant, the victim is entitled to monetary compensation to help cover the costs. Compensation can also be sought for reasons that are not necessarily economic in nature, such as the physical and psychological pain associated with a hip failure.
A division of the company Johnson & Johnson, DePuy Orthopedics is facing legal action at both the state and federal level in the U.S. in connection with the implant. Since the ASR is considered a defective product, these cases will be conducted under laws governing product liability. In the first of what may be multiple lawsuits, a California state court recently awarded more than $8 million in damages to the plaintiff. According to one estimate, as many as 10,000 recipients of the implant could have a legal case against the manufacturer.
In response to the many complaints lodged against its product, the manufacturer initiated a recall of the ASR in the U.S. in March 2010. This was after a recall had been issued in Australia, and after DePuy had announced that it would discontinue production of the ASR. However, more than 90,000 of the devices had by that time been implanted in patients around the world, with about a third of the implant operations having taken place in the U.S. Letters to members of the medical community were issued by the manufacturer the same month as the U.S. recall, and this correspondence contained information indicating that the failure rate of the ASR was higher than that of traditional hip replacement devices. The risk of failure is believed greater in women and in anyone with weak bones.
Residents of Mississippi who have experienced difficulties after receiving an ASR device should consider contacting Attorney Group for Mississippi, whose affiliated lawyers can help them seek the compensation that they deserve.





