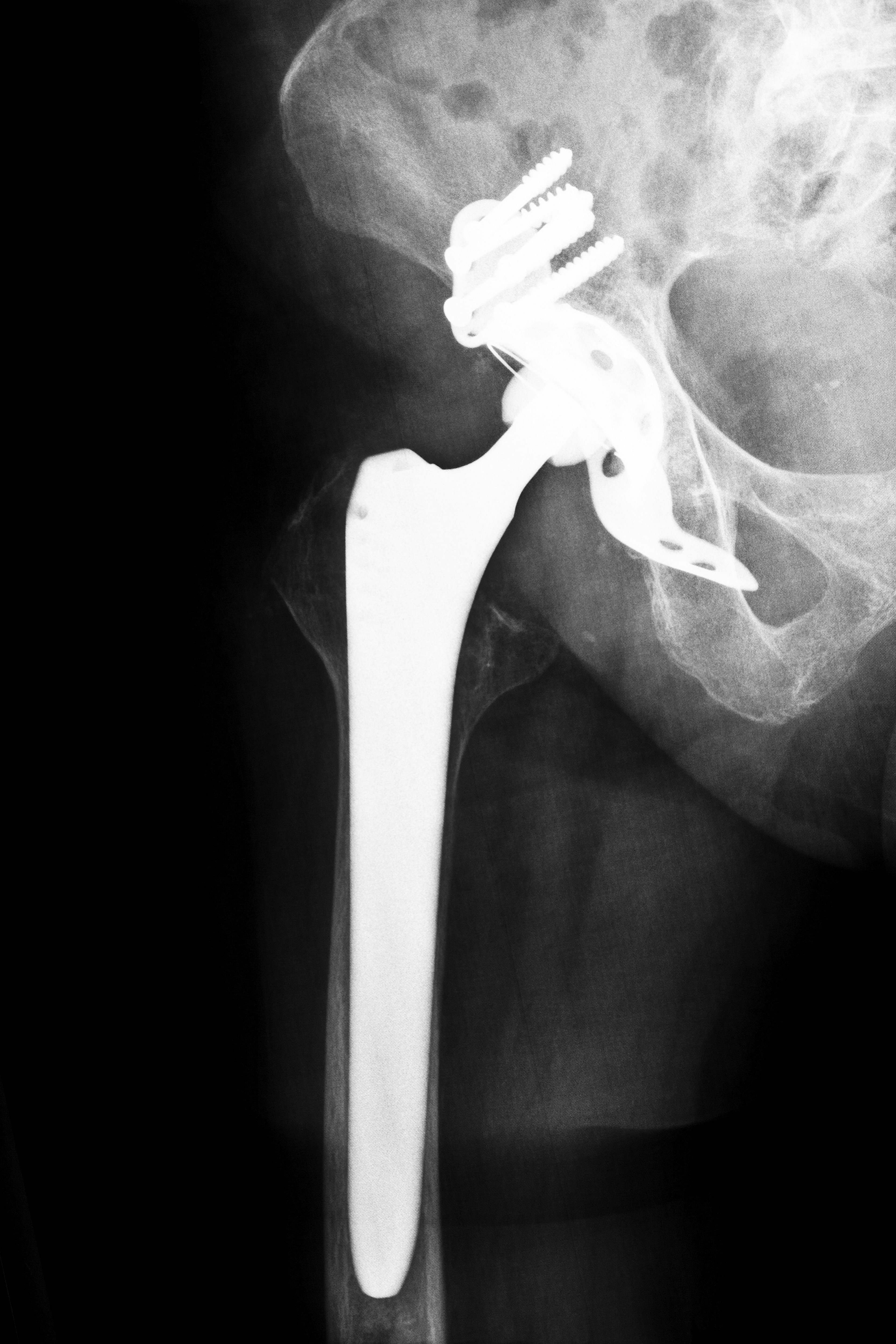
Mississippi Biomet Hip Lawsuits
Released under a special expedited process, known as 510(k), the Biomet M2A and twenty-one other metal-on-metal hip replacement devices became available on the market. Under the 510(k), the Biomet M2A was exempted from extensive clinical trials or testings because it was substantially similar or equivalent to existing products readily available.
Unfortunately, the lack of clinical trials to test the Biomet M2A meant that all of its errors, side effects, or complications were discovered by the patients themselves after hip replacement surgery. Indeed, metal-on-metal hip replacement devices have proven to be more prone to breakdown and fracture and significantly more unreliable and unsafe than their plastic-on-metal counterparts. Not long after the FDA placed the Biomet M2A Hip Replacement Device on the biomedical market, it received nearly 20,000 reports of malfunction and adverse side effects.
Like other metal-on-metal hip replacement devices, the Biomet M2A consists of a metal femoral ball portion, which grinds inside the metal socket when the patient moves. This friction can chip away at the metal ball and distribute debris into the blood stream, resulting in metal toxicity. In addition, the friction can inhibit easy mobility and break down surrounding tissue, resulting in tissue injury and death, or necrosis. These adverse side effects were documented in an FDA-sponsored panel on the risks of metal-on-metal hip replacement devices as well as studies performed by Health Canada and the British Journal of Medicine.
Complications with the Biomet M2A Hip Implants: What You Should Know
The femoral ball portion of the Biomet M2A hip replacement device is the principle source of complications. When the individual moves, friction agitates the structure of the device. Since the hip joint is essential to all movement, including walking, the breakdown of the device results in decreased mobility. Impaired mobility can result in fractures. In light of all of this, Mississippi Biomet hip lawsuits were inevitable.
If you or someone you know received a Biomet M2A hip replacement device during or after 1998, and have experienced complications since the insertion of the device, you may be eligible for compensation. Since necessary clinical trials were sidestepped to expedite its entry into the market, much information on the side effects and range of complications is currently being acquired. However, the following side effects and complications have been found to be associated with the insertion and use of the Biomet M2A hip replacement device:
- Femural bone fractures
- Damage to nerves
- Inability to walk or move
- Rashes over tissue due to tissue necrosis
- Chronic pain, particularly in joint
- Heightened and/or toxic levels of the metal chromium in bloodstream
- Inflammation of surrounding tissue
- Muscle inflammation
- Need for corrective hip surgery or hip revision surgery
- Clotting or damage to blood vessels surrounding device
Such symptoms frequently occur within 6 months to 29 months of hip replacement surgery. As a metal-on-metal hip replacement device, the Biomet M2A was included in the FDA-issued recall and this recall applies to replacement devices inserted from 1998 onward.
Current Legal Action
Current Mississippi Biomet hip lawsuits are underway as thousands of victims in Mississippi and throughout the United States have come forward to seek redress for complications. Attorney Group for Mississippi can work with you through one of its affiliated lawyers, seeking compensation through federal litigation on behalf of all Mississippi Biomet hip lawsuits and handles each case with sensitivity and individual attention. The extent of the lawsuits is staggering. Each year, approximately 200,000 hip replacement surgeries are performed. It is estimated that 5-8% of such surgeries insert the Biomet M2A hip replacement device. Biomet is one of the sever hip implant manufacturers under scrutiny for faulty hip replacement devices. Federal litigation efforts seeks to acquire compensation for victims for alleged suffering, pain, loss of employment, related medical expenses, loss of mobility, complications due to metal toxicity, and more.
Ways to Seek Legal Redress
If you or someone you know has received a Biomet M2A Hip Implant and has experienced complications or pain from the device, you may be eligible to receive compensation for pain, suffering, wages lost due to injury, related medical expenses, and more. For a confidential, free, and thorough review of your case, contact Attorney Group for Mississippi today. It is crucial to note that statutes of limitations, which place a limit on how much time an individual has to pursue compensation, may apply to some cases, so please contact us as soon as possible.