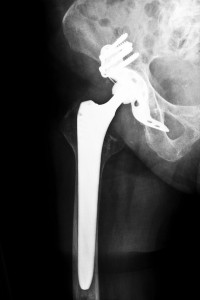
In June 2012, medical device manufacturer Smith & Nephew issued a recall of the metal cup component included in its R3 Acetabular System hip implant. According to the company, an internal investigation revealed that the product was causing a higher-than-anticipated complication rate, and reports were filed on behalf of patients who allegedly experienced early device failure. In many cases, hip recipients were required to undergo additional procedures to correct the complications that were allegedly caused by the device. Attorney Group for New York can assist R3 Acetabular System recipients in determining whether they are eligible to seek damages for their injuries by filing a New York Smith & Nephew hip lawsuit.
Safety of the R3 Acetabular System
Between the introduction of the R3 Acetabular System in 2007 and its recall in 2012, it is estimated that more than 7,700 patients were fitted with the device, many of whom reportedly suffered from fractures, infections and dislocations. Because the manufacturer only recalled a portion of the device, surgeons were still able to implant the R3 Acetabular System into patients but only if they used the device in conjunction with a non-metal liner. Patients reported several adverse and life-threatening complications, including:
- Implant loosening
- Dislocation
- Additional hip implant surgeries
- Deterioration of bone
- Inflammation and swelling
- Metal toxicity
- Severe pain
Additionally, patients have also reportedly suffered from a condition known as metallosis that develops when two metal components rub together and cause shards of cobalt and chromium to find their way into the patient’s bloodstream. If you notice one or more of the following symptoms of metallosis, consider seeking legal help to determine whether you are able to file a New York Smith & Nephew hip lawsuit to seek compensation for your injuries:
- Gastrointestinal problems
- Confusion
- Burning, numbness or tingling in the extremities
- Emotional issues
- Dizziness
- Headaches
- Infections
In 2013, after the U.S. Food and Drug Administration received reports from patients who were allegedly suffering from complications while fitted with all-metal hip implants, the agency announced that all metal-on-metal hip implant recipients should consider discussing the implant with their healthcare providers and request laboratory and blood testing to determine whether they may be suffering from metallosis.
As a result of the potential complications of the R3 Acetabular System, you may be eligible to file a New York Smith & Nephew hip lawsuit against the manufacturer to seek compensation for any injuries or complications you experienced as a result of the allegedly defective product. Contact Attorney Group for New York today to learn more about the options that may be available to you.
File Your New York Smith & Nephew Hip Lawsuit Today
If you or someone you love received the R3 Acetabular System and you suffered from adverse side effects or complications including early device failure, you may be eligible to file a New York Smith & Nephew hip lawsuit to seek compensation for your injuries. For more information and to schedule your free case evaluation, please contact Attorney Group for New York today. We can help answer your questions and connect you with an affiliated attorney who can file your New York Smith & Nephew hip lawsuit on your behalf and work to help you to recover the damages to which you may be entitled.





