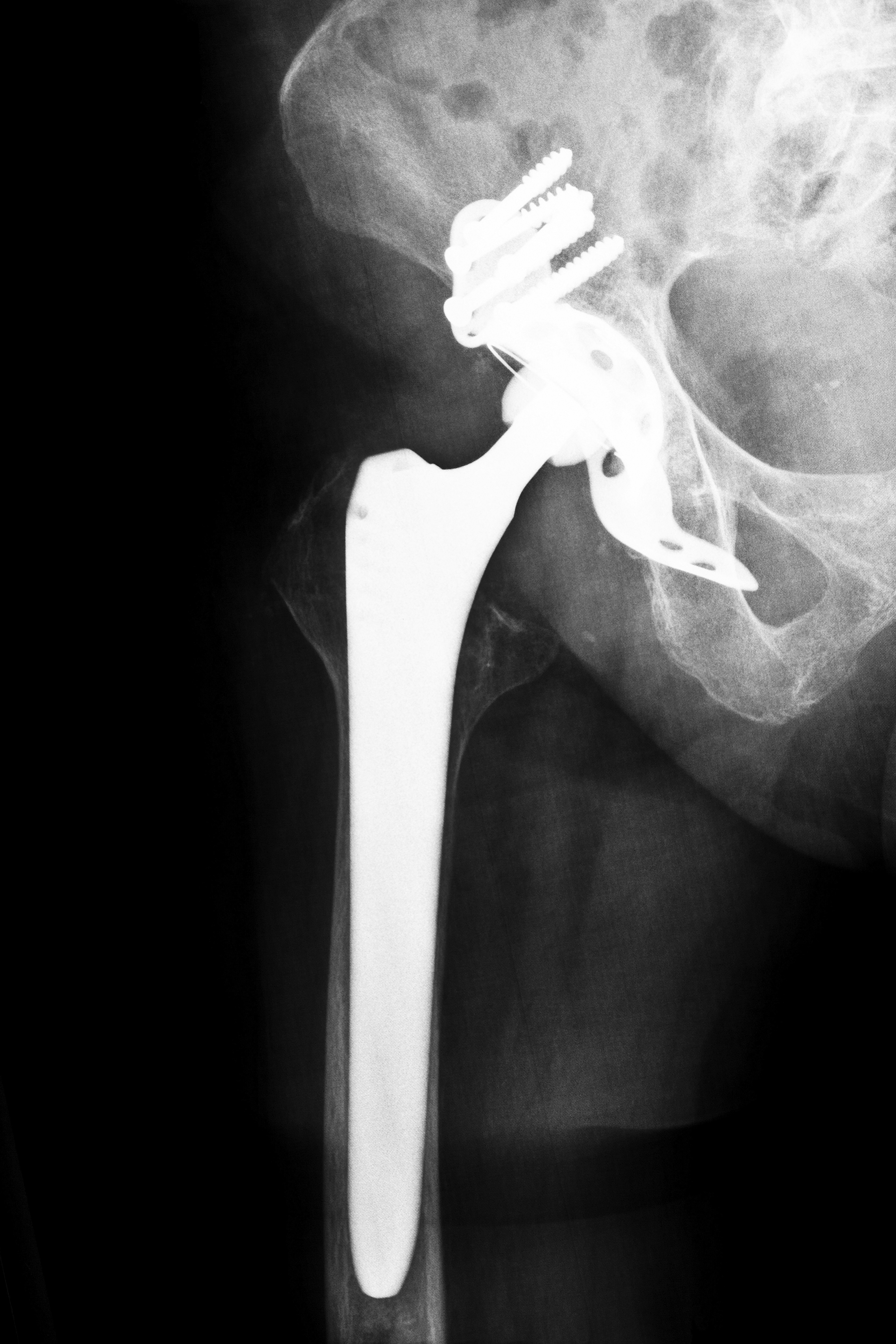
What You Need to Know about Biomet M2A Hip Implants
The Biomet M2a-Magnum Hip Implant belongs to a class of “metal-on-metal” hip replacement devices, which have come under great scrutiny in recent years. In 2004, the Biomet M2a-Magnum Hip Implant became an FDA-approved device through the controversial 510(k) procedure. The FDA’s 510(k) clause permits devices that are comparable to existing devices to enter the market without the benefit of clinical testing. Many of the defective hip replacement devices were approved through this process. Unfortunately, the Biomet M2a-Magnum Hip Implant, along with seven other hip implant devices, was recalled after more than 17,000 cases of malfunction and adverse side effects were brought to the FDA. Consequently, the FDA created an expert panel in June 2012 to assess the risks and benefits of metal-on-metal hip implants.. The panel found that the Biomet M2A-Magnum Hip Implant produced significant health risks, include risk of chromium poisoning, limited mobility, injury, and damage to surrounding tissue. In addition, Health Canada and the British Medical Journal produced studies warning consumers of the risks of metal-on-metal hip replacement implants. Consult a Tennessee Biomet M2A Hip Implant Attorney today for a free consultation or continue reading for more information.
What Are the Risks Associated with Biomet M2A Hip Implants
Unlike other hip implants, whose femoral component consists of plastic that locks into a larger metal structure, the Biomet M2A Hip Implant consists entirely of the metal. The central issue with the device is the metal femoral ball portion, which allows the hip to pivot and move. However, friction from this movements creates small chunks of metal debris, which then easily enter the bloodstream, resulting in poisoning. The femoral ball portion is partly made of chromium, which is toxic to the body in high quantities. The femoral ball is essential to movement of the entire leg and affects the whole body. Since the Biomet M2A Hip Implant was released without necessary clinical trials and tests, the following side effects have been found to be associated with the device:
- Extraordinary pain in the hip, particularly the joint
- Damage to soft tissue surrounding the hip
- Inflammation of soft tissue or muscles in proximity to device
- Need for additional hip surgery to correct device
- Metal toxicity due to increase of chromium ions in bloodstream
- Fractured bones
- Nerve damage
- Decreased mobility to inability to walk at all
- Visible rashs due to tissue death
- Tissue necrosis
- Chronic, unexplained pain
These symptoms have commonly occurred between 19-28 months after the Biomet M2A Hip Implants were inserted. The Biomet M2A Hip Implant recall applies to implants performed as early as 1998. If you are currently experiencing or have experienced any of the side effects listed above, you may be eligible for inclusion in current Tennessee Biomet hip lawsuits. 
Current Lawsuits Against the Biomet M2A Hip Implant
The Attorney Group for Tennessee is pursuing compensation through federal litigation on behalf of individuals who have experienced hip implant malfunction, loss of mobility, metal toxicity, need for hip revision surgery, pain, suffering, loss of wages, and medical expenses related to Biomet M2A Hip Implant complications. Roughly 6% of the 150,000 to 200,000 hip replacements performed have received the Biomet M2A Hip Implant, which is one of seven hip implant manufacturers whose product was part of a recall. Lawsuits allege that the metal-on-metal hip replacement device, of which Biomet M2A, has posed significant health risks to patients and is more likely to fracture, break down, require replacement, or cause serious health risks than hip replacement devices that are not made exclusively of metal. Given that the recall affects Biomet M2A Hip Implants released from 1998 to the present and impacts manufacturers of other metal-on-metal devices, the lawsuits are expected to have a broad reach and include victims nationwide. Recent litigation has resulted in settlements throughout the country. In Indiana alone, 140 lawsuits have been filed.
What Your Can Do? Consult Tennessee Biomet M2A Hip Implant Attorneys Today
If you or someone you know has received a Biomet M2A Hip Implant and has experienced complications or pain from the device, you may be eligible to receive compensation for pain, suffering, wages lost due to injury, related medical expenses, and more. Please contact Attorney Group for Tennessee for a free, confidential, and compassionate review of your case as we are currently filing Tennessee Biomet hip lawsuits. The Attorney Group for Tennessee brings to each case legal expertise and a unique passion for justice. We will do everything in our capacity to fight for your rights and provide the individual attention needed for such complex litigation. Statutes of limitations, which place a limit on how much time an individual has to pursue compensation, may apply so please contact us as soon as possible.