Tennessee Stryker Hip Recall Lawyers
Tennessee Stryker hip recall lawyers can help you if you are suffering from a recalled hip implant. Between 2008 and 2009, the Food and Drug Administration (FDA) approved the use of both the Stryker Rejuvenate and the ABG II Modular-Neck Hip Stem systems. Stryker submitted these products through the FDA’s 510(k) Premarket Notification Process which allowed the devices to be offered to the public without prior field testing or clinical trials because it had been shown that the product was similar to others already approved by the FDA. Stryker was only required to perform post-market surveillance and nothing else. In other words, the company did not have to prove the safety of the product before it was made available to the public. The flaw in this system is that problems are not discovered until after patients have been fitted with the devices.
Stryker Voluntarily Recalls Device | Call Tennessee Stryker hip recall lawyers!
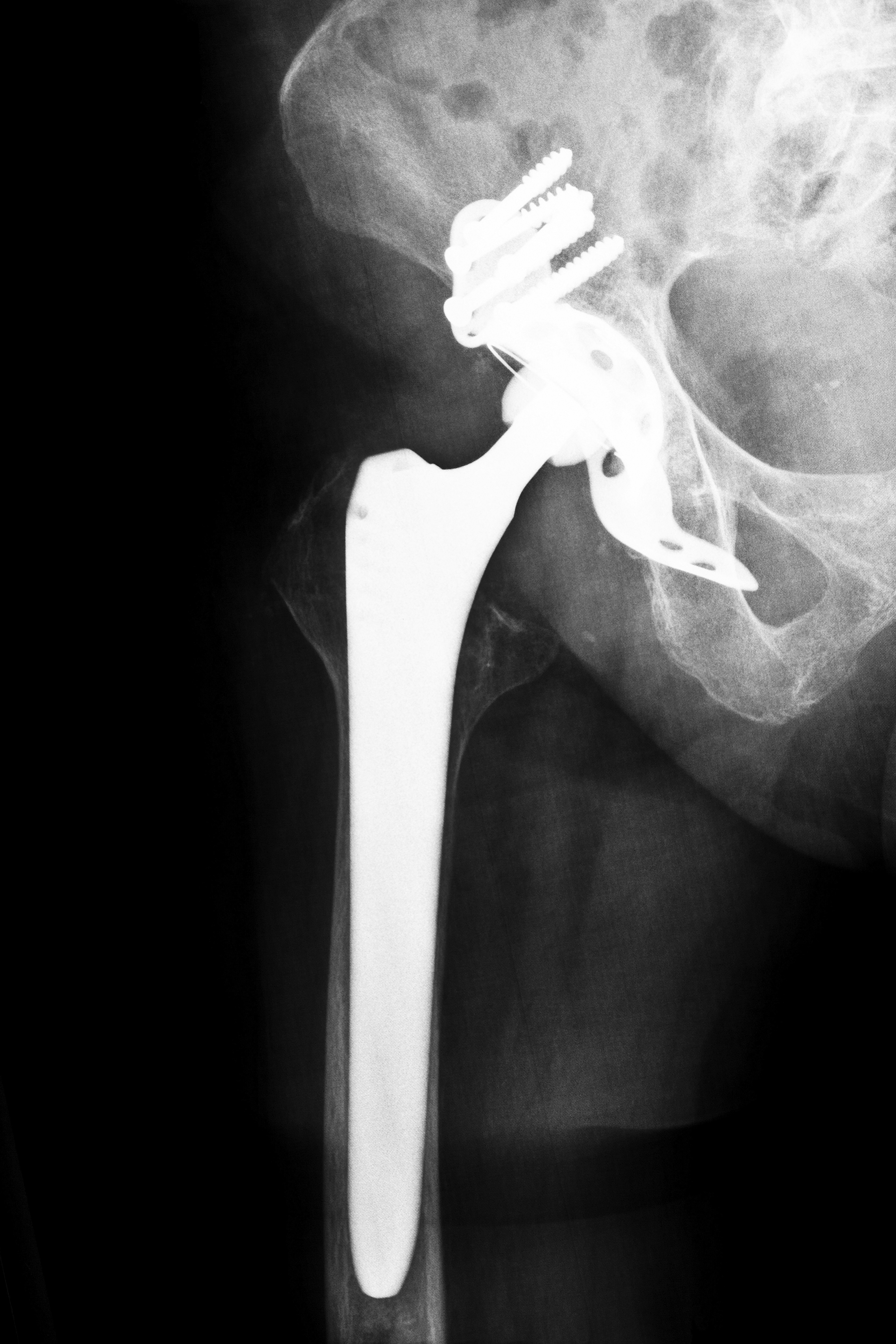
A typical hip implant consists of a two parts: a cup placed in the acetabulum of the pelvis and a femoral stem with a ball. The Stryker Rejuvenate system, however, included four parts: an acetabuluar cup, a femoral stem, a ball and a metal neck. The company promised that the extra pieces would provide longer-lasting support in its younger patients and allow the surgeon to better fit the piece into the patient. Because Stryker coated the neck with chromium and cobalt and the stem was covered with titanium, they caused similar complications as other metal-on-metal hip implant devices although they were created to replace the bone “ball-and-socket” found in a human’s hip.
Stryker warned surgeons and hospitals of the dangers of the device in April 2012, and three months later, the company initiated a voluntary recall of two of their devices, the ABG II and the Rejuvenate. They cited post-market data that showed a possibility of corrosion and rubbing of the device, causing increased swelling and pain. Medical researchers have questioned the safety of these metal-on-metal devices for years because there is always a high risk of the metal pieces rubbing against one another, thus resulting in a deposit of metal shards in the patient’s bloodstream, bones or tissues.
Treatment Options for Those with Recalled Stryker Hip Implants
In general, tens of thousands of patients are required to undergo revision surgeries to replace failed him implants. The second surgery is often dangerous and can be more complicated than the first; in fact, the mortality rate climbs from one percent to almost three percent from the initial surgery to the second. This reason alone is sufficient for any individual with a recalled hip to seek the counsel of Tennessee Stryker Hip Recall Lawyers today.
Despite the risks of receiving these devices, patients often feel they have little or no choice if they want to resume their daily lives without pain. Many surgeons agree that hip implant surgeries are difficult to perform, leaving the patient with a long road to recovery. Before the surgeon even removes the problem-causing portion of the hip, he first must dislocate the hip joint entirely. Then, he cleans out the cup-shaped cavity and lines it with a bone graft. The most difficult part of the surgery involves handling the femur, which may require yet another bone graft.
In other words, what makes Stryker implants so different from other metal-on-metal implants is that they allow the surgeon to physically correct certain aspects of the patient’s anatomy; they were created this way to offer a more personalized fit for each patients, as opposed to other companies’ hip implants that are not as easily adaptable to the individual needs of the patient.
Complications of the Stryker Hip Implants
Most patients receiving these devices are elderly and may be more prone to complications or implant failures. A positive outcome is exponentially dependent upon the quality of the defective, damaged bone. Unfortunately, while revision surgery is always an option in the aftermath of complications, it does not always fix the problem. By consulting with experienced Tennessee Stryker Hip Recall Lawyers, you can find out what clients are experiencing during revision surgery. You can also find out about the legal implications of making treatment choices. Although we advise all clients to listen to their doctors regarding health care decisions, Tennessee Stryker Hip Recall Lawyers can help in educating clients as to how each health care decision may or may not effect their legal case and obtaining just compensation for the surgery caused by the recalled hip implant device.
Some common complaints from patients and surgeons include the loosening of the implant, infection, osteolysis, hypothyroidism and necrosis. Additionally, deep vein thrombosis (DVT) has a greater chance of occurring in patients following surgery when blood clots form in the large veins in the leg. Patients may also suffer from myosistis ossification, or bone growth around the joint, leading to increased pain and stiffness.
Your Legal Options | Call our Tennessee Stryker hip recall lawyers to learn more
Stryker offered to reimburse patients who reported a failed hip implant. They are currently working with a third-party claims administrator to manage reimbursements for both the revision surgeries and other related expenses. Those who received the Stryker Rejuvenate should first consult their physician if they are concerned about possible complications.
Additionally, you may want to seek legal help from an experienced attorney. Our Tennessee Stryker hip recall lawyers will work with you to ensure that you are fully compensative for loss of income, loss of consortium and promise of income as well as punitive damages in order to discourage additional negligence. You may also be entitled to pursue a claim for pain and suffering and any related medical expenses including revision surgeries and hip implant replacements.
[maxbutton id=”1″]