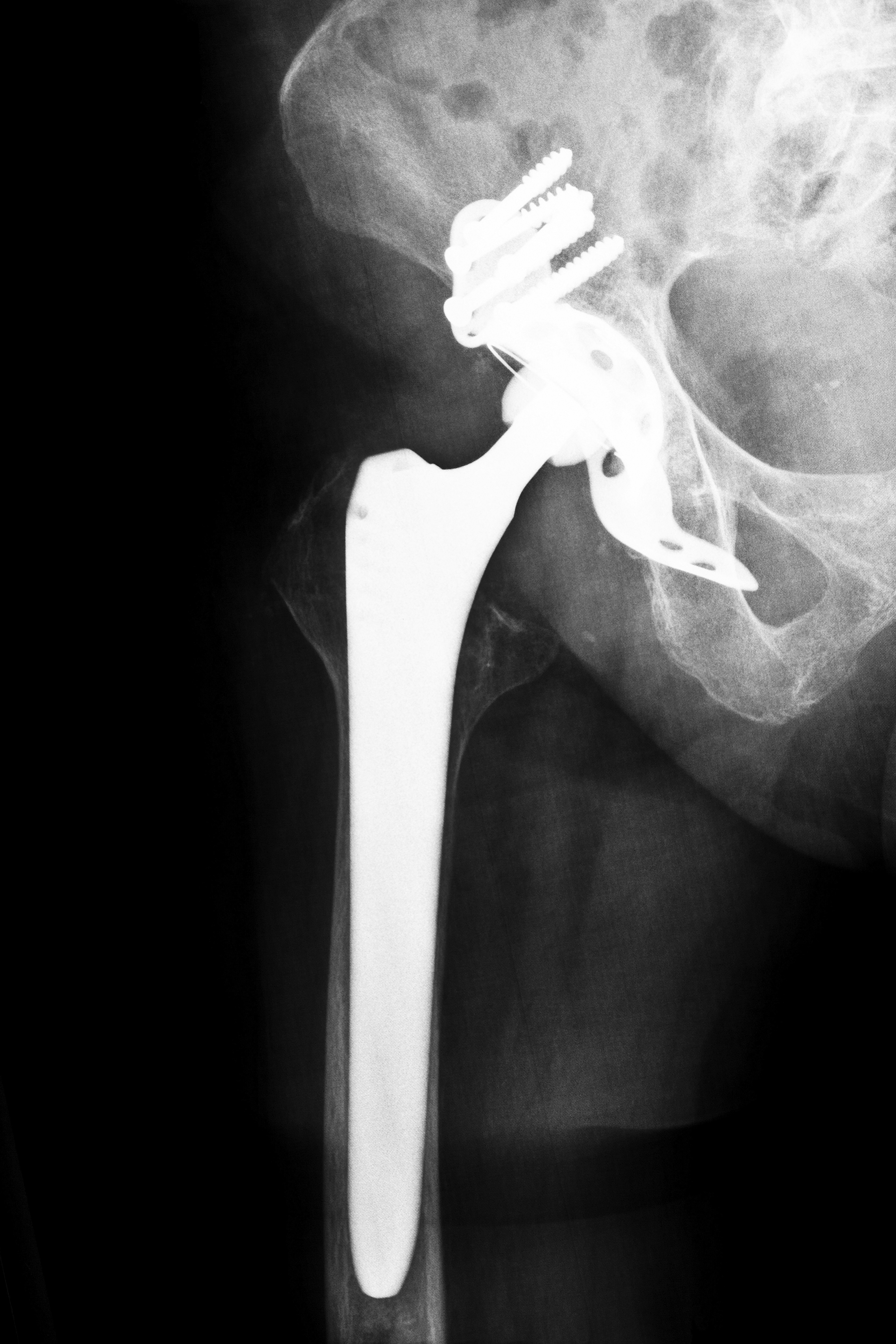
Tennessee Zimmer Durom Cup Hip Replacement Attorneys
The potential for lawsuits is being investigated by Tennessee Zimmer Durom Cup hip replacement attorneys on behalf of patients who received the Zimmer Durom Cup hip replacement and endured subsequent complications. Issues with failure of the hip replacement system after surgery led to a recall of this device in early 2008. Many physicians have reported a high number of problems with the Zimmer Durom Cup hip replacement component, which prompted excruciating pain rather than a solution for patients requiring additional surgeries.
If you or a loved one received this device, you are eligible to receive a free consultation from Tennessee Zimmer Durom Cup Hip Replacement Attorneys through Attorney Group for Tennessee.
History of the Zimmer Durom Cup
Nearly 13,000 patients have received the Durom Cup since its introduction to the U.S. market in 2006 by Zimmer, Inc., the manufacturer. This device was touted as an advanced form of artificial hip replacement systems. Originally, the goal was to prevent problems associated with traditional hip replacement systems such as limited motion range and instability. The design of Zimmer’s Durom Cup hip replacement system is made from a single material source. Metasul metal-on-metal Tribological Solution Large Diameter Heads were supposed to be compatible with the device.
Problems with the Zimmer Durom Cup
Approximately two years after entering the market, a prominent LA orthopedic surgeon noticed recurring problems that his patients were having after receiving the Zimmer Durom Cup hip replacement device. The surgeon, Dr. Larry Dorr, reported higher than expected revision rates and loosening of the components after surgery to the American Association of Hip and Knee Surgeons in April 2008. Initially, Dr. Dorr’s reports were dismissed by the manufacturer. However, in May 2008, Zimmer agreed to begin an investigation of the reported problems.
Results of investigating more than 3,100 cases led Zimmer to conclude that a higher degree of precision was required for the design and technology parameters originally set for the Durom Cup. Zimmer acknowledges that manufacturing of this device requires exceeding hip replacement techniques to achieve effectiveness. Further, the company agreed that physicians needed more training and clearer instructions before implanting the device. Some of the side effects associated with the Zimmer Durom Cup include:
- Pain
- Metal poisoning
- Instability
- Premature loosening of components
Affected patients of these injuries should seek legal advice from Tennessee Zimmer Durom Cup Hip Recall Attorneys. Consulting with experienced Zimmer hip lawyers may reveal options for recovery from these injuries.
Voluntary Suspension of Sales
After review of the cases, Zimmer decided to suspend sales of the Durom Cup hip replacement system in the U.S. because of the high failure rate with the device. Reports from the investigation showed that the device might fail to fuse properly with the patient’s bone. Instead, components had a tendency to separate and migrate within the patient’s body. When this occurs, patients experience excruciating pain that often leads to hip revision surgeries and rehabilitation.
Even though Zimmer suspended sales of this device, a recall was not issued. The stated reason for not issuing a recall was a lack of evidence that a manufacture defect or design defect was the cause. Further, the company plans to reintroduce the Durom Cup hip implant once they develop more instructions for physicians about surgical techniques that may help to avoid the problems.
FDA Missed Crucial Deadline
The Zimmer Durom Cup is considered a Class III medical device according to FDA standards. This category is reserved for medical devices that require rigorous premarket approval to include clinical trials before receiving clearance to sell in U.S. markets. However, the Durom Cup was not placed in this category. Critics, injured patients and physicians have questioned the rationale of the FDA and Zimmer, Inc. for not conducting a substantial number of premarket clinical tests.
In response to these concerns, the FDA was to develop renewed guidelines pertaining to clinical testing requirements for high-risk devices by December 31, 2012. The agency missed the deadline and it is unclear when more aggressive steps to protect the public will be taken. The FDA did issue a safety communication warning in January 2013 regarding all metal hip implants such as the Durom Cup.
In the meantime, lawsuits are being filed through Tennessee Zimmer Durom Cup hip replacement attorneys regarding Zimmer’s negligence. These lawsuits allege that the company knew–or should have known–about risks and the company’s failure to warn and educate physicians about those risks.
Help from Tennessee Zimmer Durom Cup Hip Replacement Attorneys
The Attorney Group for Tennessee believes in protecting clients from pharmaceutical companies that do not warn patients about potential risks with their devices. We believe they can be held responsible for subsequent injuries. If you or a family member has suffered an injury from the Durom Cup hip replacement system, contact us today for a free, no-obligation consultation.