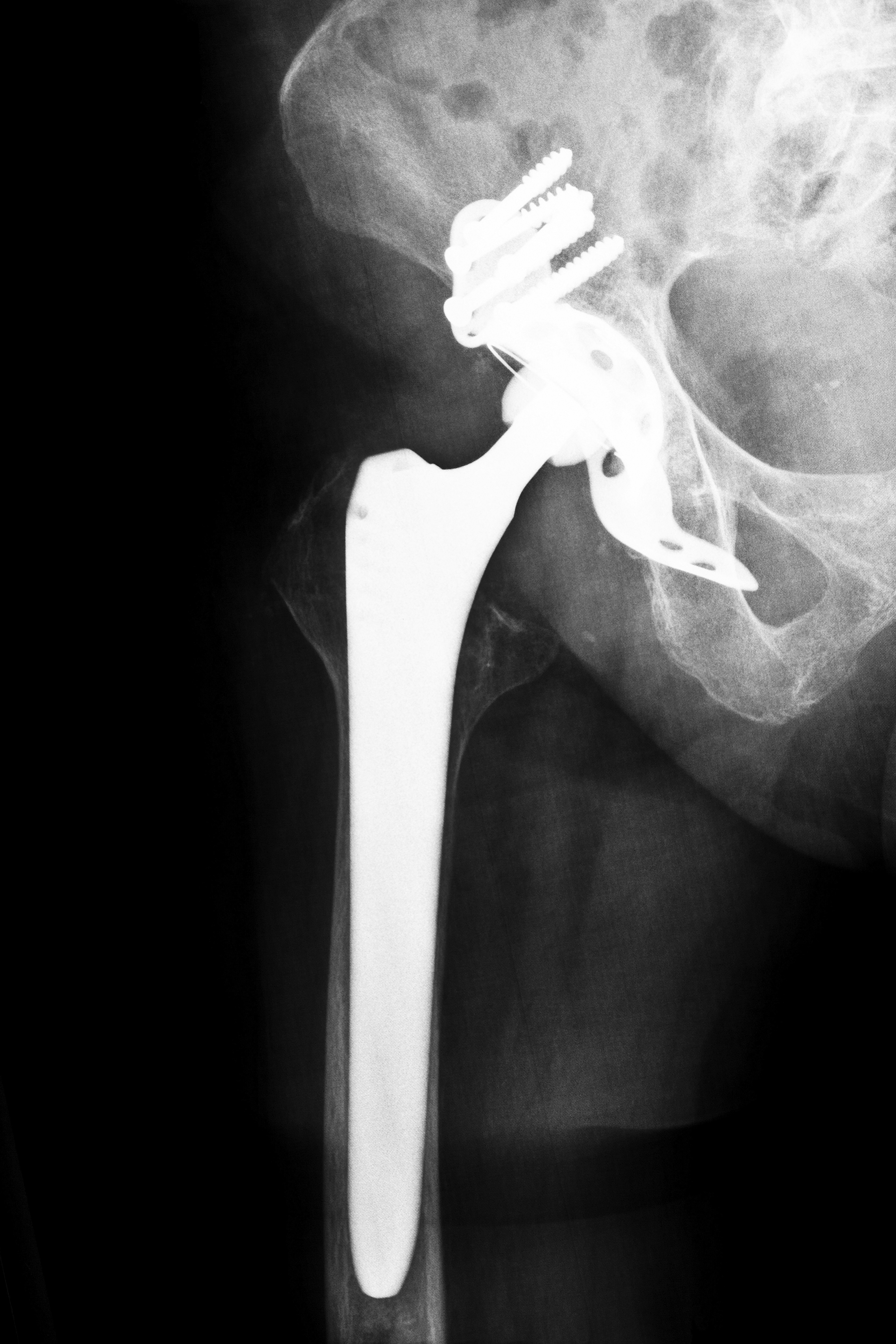
Stryker ABG II hip replacements in Tennessee causing TN Stryker Hip Recall Lawsuits
Since it was first performed in 1960, more than 200,000 Americans each year benefit from hip replacement surgery, which in most cases restores the ability to walk, sit, and stand with normal or near-normal ease. Hip implants were once thought mainly to benefit those aged 60 and over, the group most likely to suffer the stiffness and pain of arthritis. Today, however, much younger people, including athletes, undergo hip implants to restore the freedom of movement that was restricted by injury or the constant pressure and pounding of professional and amateur sports. If you have immediate questions about TN Stryker Hip Recall Lawsuits, you can skip this article and call now or fill out a form on our contact us page.
Hip replacement surgery is a great success story in America, and when it works, as is usually the case, it works very well. Some 80% of these surgeries are successful, allowing people to walk without pain or stiffness and to return to normal activities. Unfortunately, in some cases, the implant does not work. Instead, it causes pain, stiffness, swelling, and eventually the need for a second surgery, which is not only expensive but carries higher risks than the initial operation.
In 2012, Stryker Orthopaedics in Michigan, a leading medical technology firm, recalled two of its artificial hip implants, the Rejuvenate and the ABG II. The recall was voluntary, but forced by some 45 complaints from patients who spoke of stiffness, pain, and swelling in the area since the initial replacements.
Hip replacements mimic the human hip joint, one of the body’s largest, which has three parts in a “ball and socket” design. The implant is intended to work as naturally as the original joint. In the early hip replacements, the artificial joint was made of ceramic or plastic. Stryker’s Rejuvenate and the ABG II, however, are MoM (metal on metal devices, cited as superior to earlier models because of the ease of fit that is available with its use. However, reports began to surface about both models. The ABG II, on the market since 2009, is a plastic-ceramic design, but two of its parts are metal on metal. With use and constant motion, the metal parts tend to rub against each other, depositing tiny shavings into the patient’s body and causing metal corrosion. That results in pain and swelling in the area as well as impaired ability to sit, stand, or walk. Although Stryker commented that the incidence of complaints was still very low, the company did advise patients who had been implanted with the ABG II to be medically evaluated, including tests to determine serum metal ion level and metal hypersensitivity.
The Stryker recall is not the first concerning MoM replacement devices. Johnson & Johnson recalled two of its MoM implants, from its DePuy Orthopaedics division, in 2010. The complaints were the same as those that forced the Stryker recall.
Anyone who has had an ABG II Stryker implant and is suffering any distress since the surgery should become educated about the pending TN Stryker Hip Recall Lawsuits. Contact an attorney in Tennessee, who will study your case to determine the legal rights that can bring compensation for your medical expenses as well as pain and suffering and lost wages. Since the recall, there have been a number of Stryker hip replacement lawsuits. Your lawyer will be experienced in the rules and regulations that govern such lawsuits in the state and will study your case on a contingency basis, meaning without charge, including any fees, until it is won.
If you suffer in any way from the ABG II implant, participation in the TN Stryker Hip Recall Lawsuits is the best way to find justice in this battle. Your attorney needs to prove one thing only to win your case. Your condition today, whether an inability to walk, stand, or sit without pain and suffering, is directly the result of the ABG II implant. Call an attorney in Tennessee today.