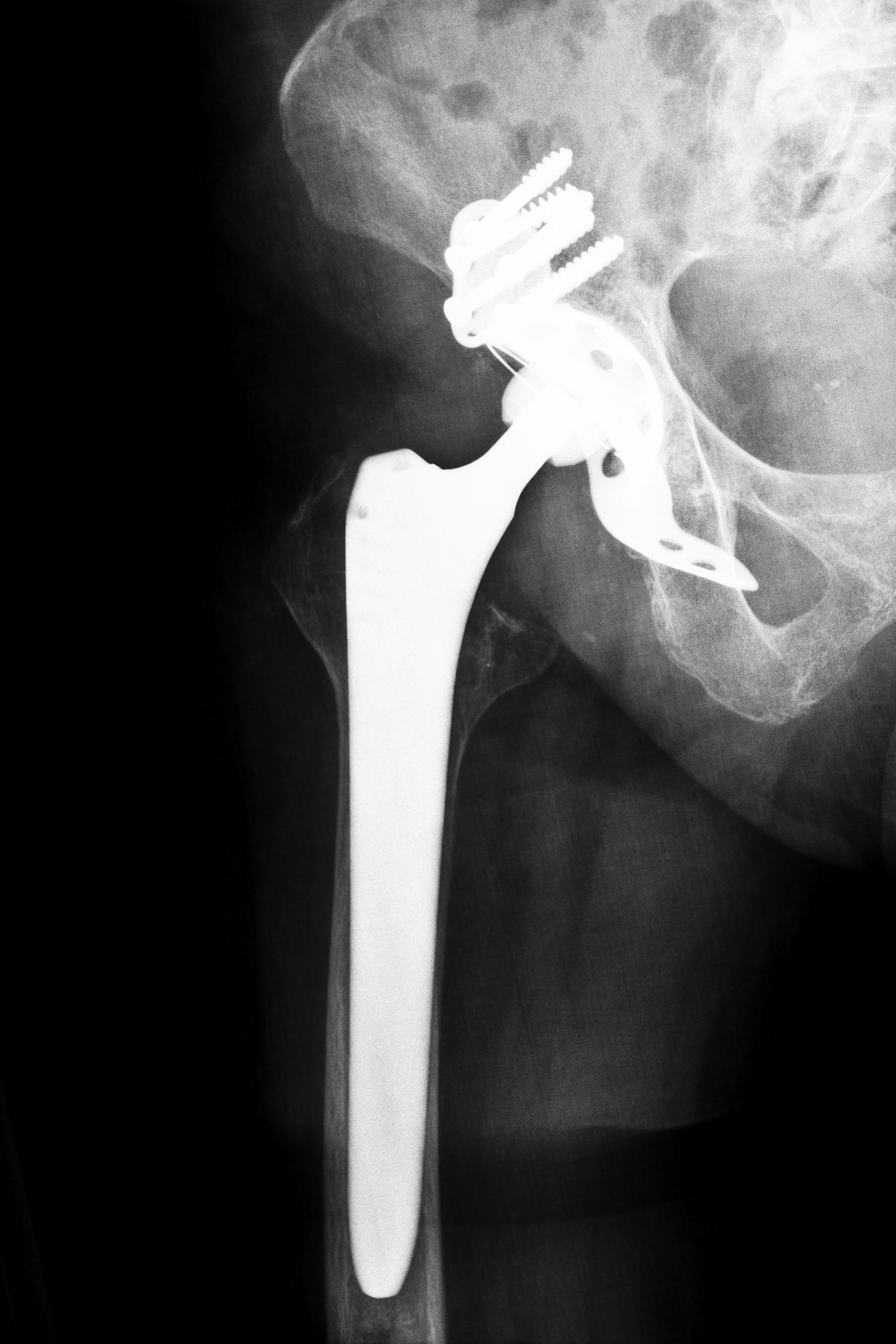
Many Colorado residents, or their loved ones, have had hip replacements. With all the publicity surrounding problems with some types of metal-on-metal hip replacements, people may find themselves confused. In many instances, doctors are as confused as their patients regarding the issues surrounding these hip replacements, so it’s important to obtain accurate information.
Reported Problems With 
Metal-On-Metal
Most recent problems, leading to recalls ion some instances, have been with Metal-on-Metal (MoM) devices used in total hip replacements. MoM devices replace both the ball and socket of the hip joint and were intended to last longer and keep the hip joint stable because of the larger size of the ball used to make the joint (http://orthoinfo.aaos.org). They were also designed to avoid debris issues that developed with earlier, plastic hip replacement systems. However, in many instances, this wasn’t the case.
Many patients with MoM hip replacements have experienced hip and groin pain, localized swelling, numbness or changes to their gait. These symptoms alone are not indicative of a faulty device, but if you also experience infection in the area, bone loss, joint loosening or a dislocation, you should check with your hip replacement surgeon to determine if you have a recalled device (www.fda.gov).
Many MoM hip replacement systems were recalled because the wear and tear on the metal devices can cause tiny pieces of metal debris, which accumulate in the joint space. As the metal corrodes, metallic ions can enter either the joint capsule causing inflammation or the bloodstream, causing an infection (http://orthoinfo.aaos.org). Other side effects include cardiomyopathy, neurological impairments, renal dysfunction or hypothyroidism (www.fda.gov).
While the FDA offered guidance to the public and ordered a recall of certain MoM hip replacements, by the time of these actions nearly half a million Americans had received the problematic and recalled hip replacements. The FDA has not issued a recall for all MoM hip replacements, despite complaints showing that many MoM devices are subject to the metallic wear and tear issues reported to the FDA by consumers and physicians.
The manufacturers of recalled hip replacements include (www.drugwatch.com)
- DePuy Articular Surface Replacement (ASR) Acetabular Hip System (2010 recall)
- Stryker Rejuvenate and ABG II Hip Systems (July, 2012 recall)
- Smith & Nephew R3 Acetabular System (June, 2012 recall)
Compensation paid by manufacturers in connection with their recalls may not cover future medical expenses or payment for lost wages. Additionally, many manufacturers suggest getting a revision surgery, which involves removing one implant and replacing it with a new one. Revisions surgeries are often more involved and patients can have more pain and a longer recovery time.
Problems with a hip replacement? Call Us Today.
Colorado residents struggling with recalled hip replacements, or those who have a loved one in Colorado and wonder if they are affected by the recalls, should contact Attorney Group for Colorado to learn more. There is no cost or obligation to speak to us, and if you have a claim we connect you with an affiliated attorney who can assist you with pursuing compensation for damages caused by your recalled hip.