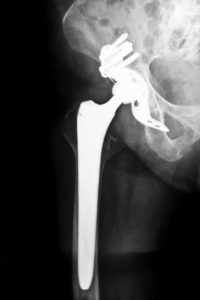
Patients who have experienced complications while fitted with a Biomet M2a Magnum hip implant may have legal recourse. According to lawsuits currently proceeding across the U.S., the M2a Magnum was allegedly defective and caused severe and life-threatening complications. Victims are pursuing claims for pain and suffering, medical expenses, lost wages, and other damages stemming from their injuries. For more information about filing your own Colorado Biomet hip lawsuit, contact Attorney Group for Colorado today for a free, no-obligation consultation.
Plaintiffs Pursuing Biomet Hip Lawsuits in the U.S.
According to plaintiffs, the two metal components of the Biomet M2a Magnum can potentially rub together while the patient is walking, standing, bending, or performing other daily activities. Patients may develop metallosis, or metal toxicity, if the friction causes metal to be ions released into the blood stream. If you have suffered from one of more of the following complications, contact Attorney Group for Colorado for more information about filing a Colorado Biomet hip lawsuit:
- Bone disintegration
- Chronic pain
- Permanent disability
- Tissue death
- Formation of pseudotumors
- Early device failure
- Removal and revision surgeries
- Metal poisoning
According to media reports, the U.S. Food and Drug Administration (FDA) has been investigating whether metal-on-metal hip replacement devices such as the Biomet M2a Magnum are safe. The FDA released a public announcement in January 2013 warning consumers of the potential for tissue and bone damage if the all-metal components rub together. Those allegedly injured by the device are urged to consult with Attorney Group for Colorado to determine whether they are eligible to file a Colorado Biomet hip lawsuit.
The FDA also suggested that every patient who has received a metal-on-metal hip implant undergo imaging and blood testing to determine whether they are suffering from metallosis, regardless of whether they notice symptoms. The agency is in the process of proposing a new regulation that would prevent these metal-on-metal devices from receiving approval through the FDA’s 510(k) clearance process. This process allows products to be released for use without clinical testing if manufacturers can prove that their new devices are similar in function and design to a product currently on the market.
While removing all-metal hip implants from 510(k) eligibility may be a wise decision, it does little to help those who are already experiencing adverse side effects and complications. Patients who believe that their hip implant caused them to sustain serious and life-threatening injuries may be eligible to file a Colorado Biomet hip lawsuit and recover damages for their injuries. For more information about your options, contact Attorney Group for Colorado today.
Eligible to File a Colorado Biomet Hip Lawsuit?
If you were fitted with a Biomet hip replacement device and experienced complications as a result, you may be eligible to file a Colorado Biomet hip lawsuit. Attorney Group for Colorado is currently offering free, no-obligation consultations to help patients determine whether they have a claim. We can also connect you with an affiliated attorney who can file a Colorado Biomet hip lawsuit on your behalf and assist you in seeking the compensation to which you may be entitled.





