
A Washington Onglyza lawsuit may be an option for diabetes drug patients who have suffered heart failure as a result of taking Onglyza. The diabetes medication has been linked to serious side effects, including those affecting the pancreas, thyroid, and cardiovascular system. Affected patients and families may be eligible to file a Washington Onglyza lawsuit and pursue compensation for damages allegedly associated with the medication.
For more information, contact Attorney Group for Washington. We offer free, confidential, no obligation consultations. We can help answer your questions, and if you choose to pursue a case we can connect you with an affiliated Onglyza lawsuit attorney who can assist you throughout the legal process.
Type 2 Diabetes Complications
Living with type 2 diabetes often requires a lifestyle change or at least changes in eating habits, exercise or medication. These adjustments are necessary because left untreated, type 2 diabetes can lead to serious conditions and even death. Factors such as genetics, extra glucose and poor cell communication contribute to type 2 diabetes, and kidney damage and nerve damage are just two of the possible complications, as the American Diabetes Association explains.
Stroke is another possible complication, especially if people are smokers, overweight or have heart disease. When compared to people without diabetes, the risk of diabetics having a stroke is one and a half times more likely. People with diabetes are also in danger of foot numbness. They may get a serious foot injury and not realize it for quite some time because their feet are numb.
What is Onglyza?
Onglyza, a diabetes medication developed and distributed by AstraZeneca, is one of several DPP-4 inhibitors used in the United States, according to the American Diabetes Association. While other diabetes drugs may cause increases in cholesterol and weight, DPP-4 inhibitors show either no influence or positive influence in these two categories. These inhibitors help the body decrease glucose levels by forcing the GLP-1 compound to work longer.
According to the official website for Onglyza, the drug is not to be used with people experiencing diabetic ketoacidosis. It is also not for use in type 1 diabetes. Side effects include headache and upper respiratory tract infections. Severe side effects of the medication include worsening fluid retention, joint pain, an increased risk of hypoglycemia and pancreatitis. Heart failure is also a potential complication.
Onglyza Risks and FDA Warnings
In addition to common side effects, the U.S. Food and Drug Administration (FDA) has released the following warnings and safety communications regarding the use of Onglyza and serious, potentially life-threatening risks including:
- Pancreatitis and Pancreatic Cancer Risks: According to a warning issued by the FDA in 2013, acute pancreatitis was reported in patients taking Onglyza. If pancreatitis is suspected, patients should stop using the medication and inform their doctor as soon as possible. In addition to risks of pancreatitis, there is a potential for the increased risk of pancreatic cancer from DPP-4 medications, including Onglyza.
- Cardiovascular Risks and Heart Failure: In April 2015, an FDA advisory committee voted to recommend that the drug’s label include new information warning patients about the potential risks of heart disease associated with the use of Onglyza. Based on a study of over 16,000 patients with type 2 diabetes, results published in the New England Journal of Medicine noted that Onglyza patients were more likely to be hospitalized with heart failure than those who did not use the medication. Following the study, the FDA asked that the manufacturers of Onglyza present clinical trial data so that they may look into a possible link between the use of the medication and heart failure. Although the investigation was preliminary, it was suggested that patients speak with their healthcare provider if they had any concerns.
However in April 2016, the FDA issued a safety announcement stating that after a safety review, the agency found that type 2 diabetes medicines including Onglyza, containing saxagliptin and alogliptin, may increase the risk of heart failure, particularly in patients who already have heart or kidney disease. Heart failure can result in the heart not being able to pump enough blood to meet the body’s needs. As a result, the FDA added new warnings to the drug labels about this safety issue.
Joint Pain: In a 2015 FDA safety communication, the agency announced that the use of DPP-4 inhibitors, including Onglyza, may cause severe joint pain. The warning advised patients taking DPP-4 inhibitors to notify their doctors immediately if they start to develop persistent joint pain while taking Onglyza.
How a Washington Onglyza Lawsuit Can Help
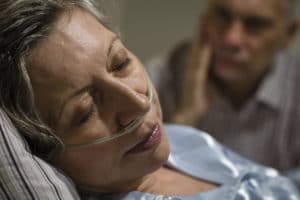
Drug makers have a duty to provide safe products. If there are risks of harm associated with their products, they also must provide adequate warnings. If a drug maker fails to fulfill this duty, it could be held liable in lawsuits for injuries that may result.
People injured by Onglyza may be eligible to recover money for:
- Medical Expenses
- Lost Wages
- Pain and Suffering
The families of those who have died may be eligible to recover money for funeral expenses and the pain that comes with losing a loved one.
The Time You Have to Pursue a Claim is Limited. Contact Us Today.
For more information, contact Attorney Group for Washington. You can fill out the form on this page or contact us by phone or email.
After you contact us, an attorney will follow up to answer questions that you might have. There is no cost or obligation to speak with us, and any information you provide will be kept confidential.
Please note that the law limits the time you have to pursue a claim or file a lawsuit for an injury. If you think you have a case, you should not delay taking action.





