 In light of recent criticism of the United States Food and Drug Administration (FDA)’s approval process, the governmental agency has released updates to their approval process to better protect the public from defective medical devices. According to a report published in the Archives of Internal Medicine in 2011, most medical devices recalled by the FDA were approved through an expedited process or were exempt from regulatory review.
In light of recent criticism of the United States Food and Drug Administration (FDA)’s approval process, the governmental agency has released updates to their approval process to better protect the public from defective medical devices. According to a report published in the Archives of Internal Medicine in 2011, most medical devices recalled by the FDA were approved through an expedited process or were exempt from regulatory review.
In some cases, the lack of oversight in approvals at the FDA has been attributed to serious injury and death throughout the United States. In August 2010, an Alabama resident suffered a heart attack and died during dialysis treatment after being administered GranuFlo, a medical device that has since been recalled. Internal memorandums suggest that the FDA and the manufacturer, Fresnius, were aware of the increased risk of cardiac arrest through the improper use of GranuFlo.
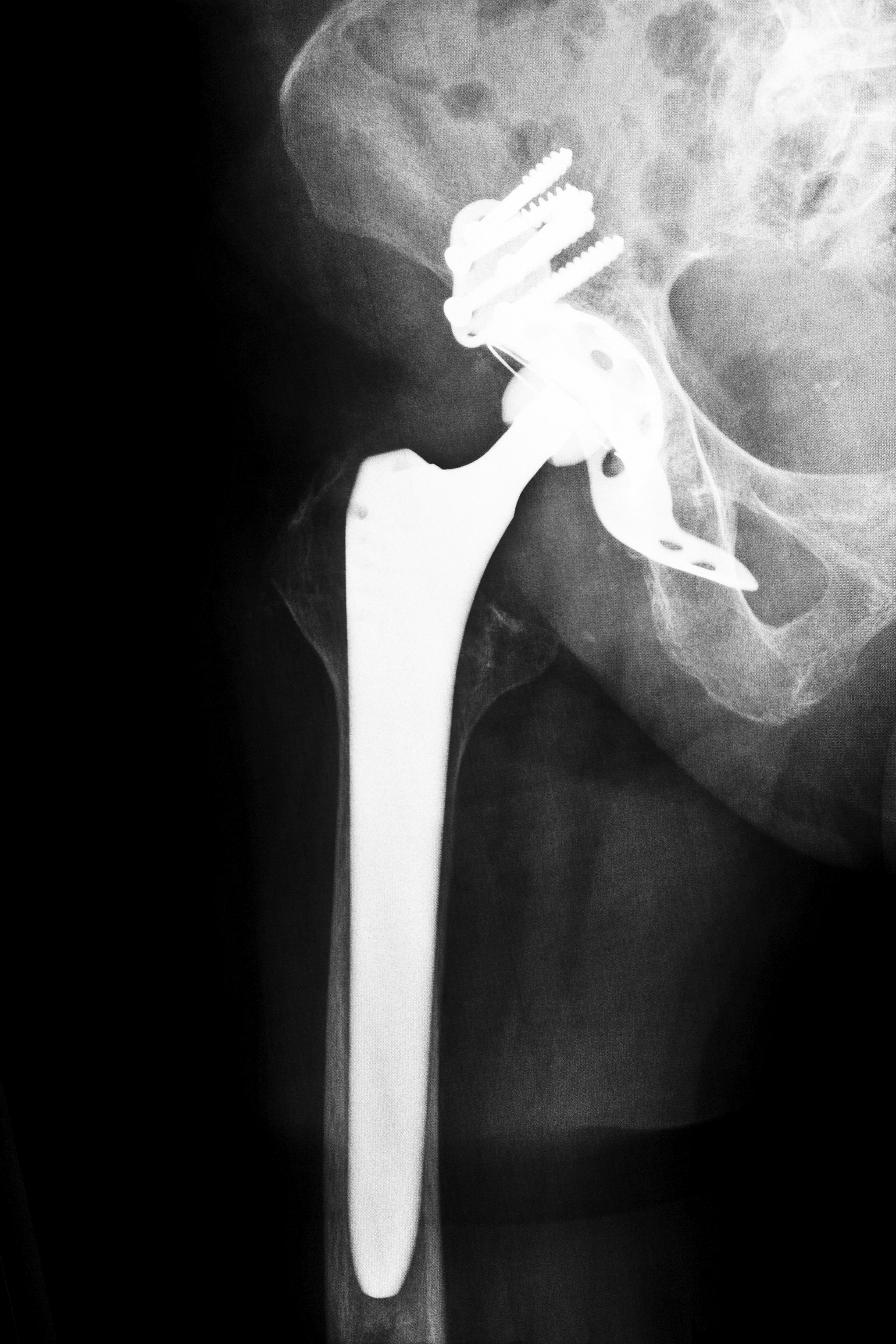
Other cases in Alabama regarding defective medical devices include injuries sustained after transvaginal mesh surgeries and joint replacement problems after faulty hip replacement devices were implanted. Despite the fact that these devices allegedly caused injury and death in Alabama and other states, the FDA reportedly faces pressure from medical device manufacturers to speed up the process of approving devices.
In April 2014, the FDA announced that an Expedited Access Premarket Approval Application program could be implemented that would speed up approvals and help patients gain access to new products more quickly. The new program allows devices that feature breakthroughs in technology that have significant benefits over what currently exists for patients.
Patient advocates do not support the new program, claiming that approval for medical devices is already significantly less stringent than those required for prescription drugs. It is believed that the one percent of devices that will be processed through the expedited program are some of the most risky as they sustain human life, prevent impairment of human health or present an unreasonable risk of illness or injury. Experts also say that the less stringent rules define “urgent, unmet need” too loosely. This could mean that accelerated access to defective medical devices could lead to injury or death needlessly.
Do You Have a Defective Medical Device Lawsuit?
If you or a loved one has been injured by a defective medical device, contact Attorney Group for Alabama today to learn more about a potential product liability claim. We can help you understand your options in your unique situation, and connect you with an affiliated Alabama attorney if you have a case. Contact us to learn more in a free consultation.