Alabama Hip Replacement Lawsuits Filed in AL
The advances in modern medicine mean people suffering from osteoarthritis have the opportunity for obtaining relief from the debilitating effects the disease process presents. Prosthetic implants replace damaged and disease-ridden joints. However, in recent years, implant manufacturing companies, and Stryker in particular have caused numerous arthritis patients additional pain and suffering from defective products. Thousands received the Rejuvenate Hip Stem systems. Defects in the product came to the attention of the FDA. Though a recall began, Alabama Hip Replacement Lawsuits emerged. Alabama Stryker Hip Recall lawyers remain involved with clients enduring the agony of having a faulty implant.
Arthroplasty
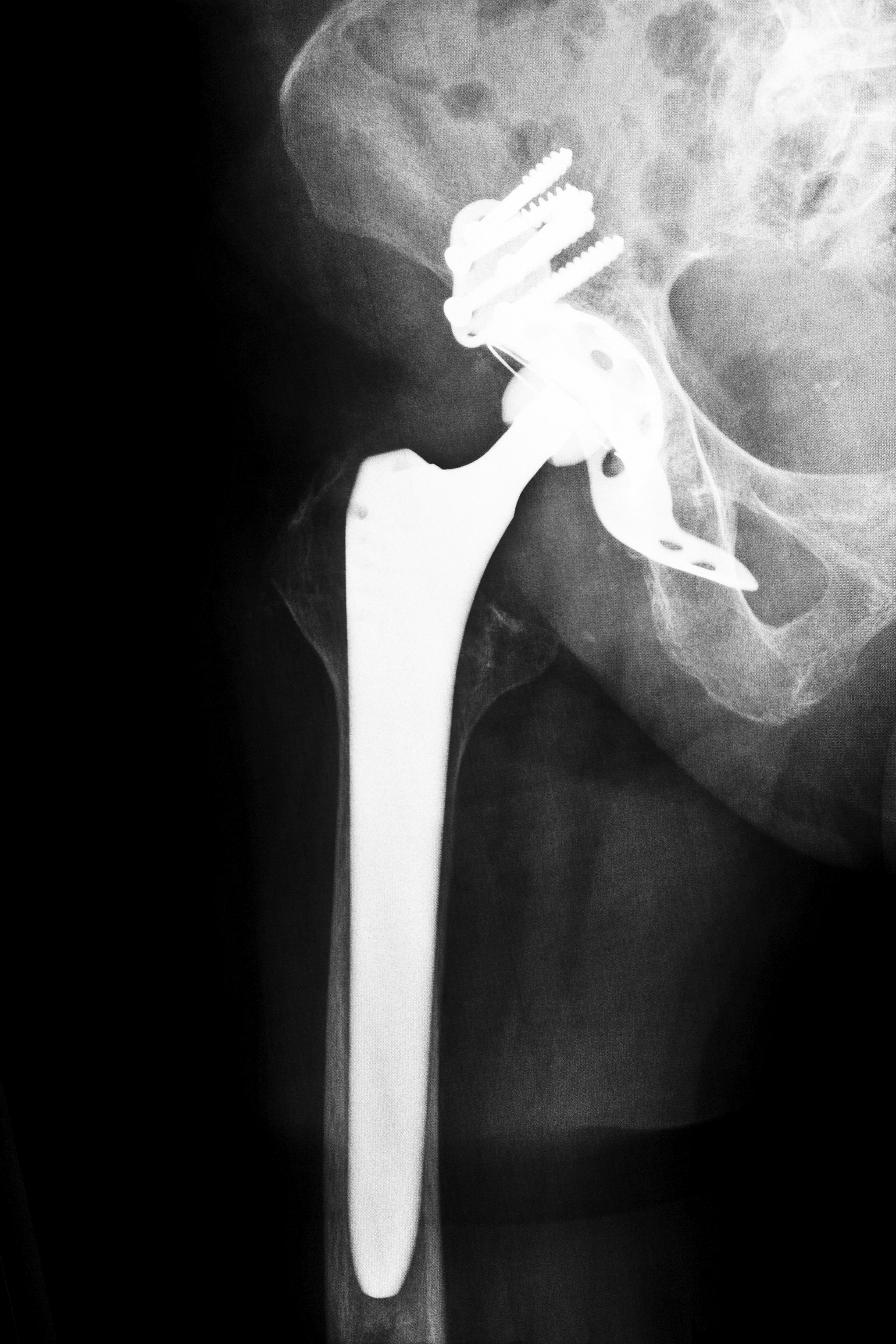
Hip replacement surgery or athroplasty involves removing the diseased bone and connective tissue. Surgeons use synthetic ball and joint implants as a means of curing the damage. The Stryker hip replacement system consists of four separate pieces that include stems, necks, balls and cups that comprise the complete joint. Manufactured in varying sizes, the company designed the system so surgeons might more easily mix and match components, creating individual systems that accommodate each patient. However, the number of movable parts of the system increases the likelihood for malfunction. 
Malfunctions Associated with the Stryker Rejuvenate Implants
Problems began with the ceramic base of the components. Patients reported hearing squeaking noise when the joint underwent normal movement, as the ceramic parts made contact. The components additionally have a chromium and cobalt coating created to resist corrosion and damage from movement. However, during joint extension, flexion and rotation, the metal coatings between the ball and socket make contact, which degrades the coating. This contact poses metal-on-metal problems associated with older model devices.
Patients began suffering from a diagnosis known as Adverse Local Tissue Reaction, as the metal coating deteriorated. The microscopic particles or shavings not only cause localized trauma but additionally create a toxic condition known as metallosis. The problem develops gradually and patients initially do not experience symptoms. When undiagnosed and untreated, the particles cause tissue death within the bone, connective tissues and muscles. The bones weaken and fracture becomes a possibility. The minute fragments might also enter the bloodstream, which results in metal poisoning. The irritation may also cause growths similar to tumors. Patients suffer a wide gamut of symptoms ranging from allergic reactions to immunological abnormalities. In rare instances, the condition causes death.
Patients having Rejuvenate implants begin noticing bruising, reddening and swelling around the joint area accompanied by discomfort to extreme pain. In time, the tissue degradation may cause dislodging of the implant. Physicians diagnose the condition through blood testing and imaging studies. If blood tests reveal positive for traces of metal, the physician makes a determination of metallosis. Metallic levels measuring seven parts per billion cause toxicity.
Patients having malfunctioning implants require corrective or revision surgery. Besides having to endure a second surgery, the removal process remains complicated. Bone and connective tissue normally cover the implant, and device removal causes tissue trauma. The stem lies deeply embedded within the femur and bone weakening may cause fractures. Osteopathic surgeons must then perform repairs on the fracture, or fractures, before installing a new implant. The additional trauma extends the recovery process compared to the recovery experienced after the initial surgery.
Pre-Market Notification Process
The Stryker Rejuvenate implant received FDA approval without undergoing an extensive testing process through clinical trials. The 510(k) Pre-market Notification Process approves medical products when similar designs have current administrative endorsement. The FDA did not consider the fact that older implants having metal exteriors presented complications. Subsequently, product defects and problems did not emerge for the Rejuvenate system until after patients received the implants.
Physicians practicing in Australia first encountered problems with the devices. The country’s joint replacement registry provides tracking of each medical implant. By 2012, the FDA received dozens of complaints concerning the Rejuvenate and another Stryker systems. The company initiated a voluntary recall. The Alabama Hip Replacement Lawsuits continued and Stryker alerted medical facilities and surgeons of the safety concerns.
Alabama Hip Replacement Lawsuits Should be Filed for Implant Trauma Compensation
Stryker remains involved in a number of lawsuits filed against the Rejuvenate systems. The suits charge the company with negligence secondary to a lack of adequate testing and not informing consumers and physicians concerning adverse reactions and defects associated with the implants. While the company willingly offers compensation for testing and implant removal, patients have not received settlements regarding any long-term problems that accompany metallosis. Anyone having problems with a Stryker Rejuvenate implant should contact their health care physician and a Stryker hip recall lawyer regarding the Alabama Hip Replacement Lawsuits. Trained and experienced in personal injury, attorneys review individual cases and inform clients of their legal rights.