 Progress is being made in finalizing a Biomet settlement in the U.S. District Court for the Northern District of Indiana. There, more than 2,400 complaints are being processed as multi-district litigation (MDL). On September 17, 2014, a joint status report was released that details the progress that is being made ever since a preliminary agreement was announced in February 2014. Some effected parties in Alabama have been following this case.
Progress is being made in finalizing a Biomet settlement in the U.S. District Court for the Northern District of Indiana. There, more than 2,400 complaints are being processed as multi-district litigation (MDL). On September 17, 2014, a joint status report was released that details the progress that is being made ever since a preliminary agreement was announced in February 2014. Some effected parties in Alabama have been following this case.
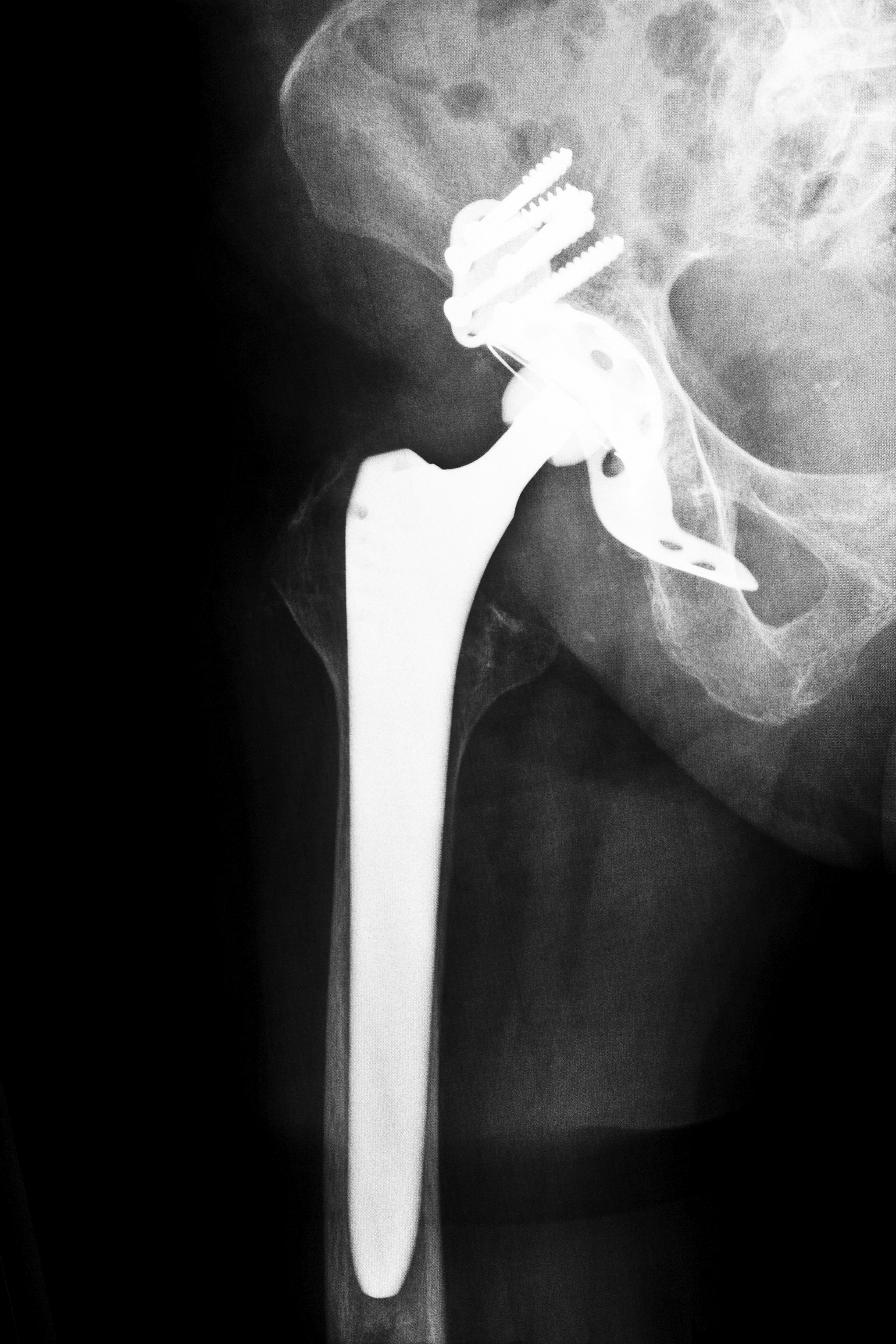
Biomet Hip Problems Alleged
Biomet lawsuits followed problems that allegedly occurred after Biomet hip implant surgery in Alabama and elsewhere. Some patients allegedly experienced severe pain, and hip revision surgery was required only months after the initial procedure. Some patients allege in their complaints that metal-on-metal contact allegedly resulted in the release of metal particles into surrounding tissue or into the bloodstream. This allegedly resulted in metal poisoning in some patients. Suits have also alleged that Biomet Orthopaedics continued to represent its hip implants as safe and effective even after numerous problems had been reported.
Settlement Fund Created for Biomet Settlement
Judge Robert L. Miller has presided over the case in the Federal Court in Indiana. He has ordered that a PEC Biomet Qualified Settlement Fund be created, per Treasury Regulation Section 1.468B-1. Judge Miller has designated PrivateBank and Trust in Chicago as the institution that will hold the funds, and he has named Garretson Resolution Group, Inc. as the fund’s administrator.
Each complainant in this Biomet settlement will receive at least $200,000 from the settlement fund, according to the joint status update. However, individual complainants may request mediation in order to seek higher payouts when certain variables exist. Some individuals have already entered into mediation. It is estimated that the total awards in this Biomet settlement will exceed $100 million. Biomet Orthopaedics had previously deposited $50 million in an escrow fund that will be used to begin to pay litigants.
Specific Patients Included
It is important to note that the litigation in the Federal court in the northern Indiana district only applies to patients that specifically received an M2a 38 or M2a Magnum Biomet hip. Plus, these hip implant recipients must have had hip revision surgery with 180 days of their original surgery. There is also a provision in the pending Biomet settlement agreement that addresses the needs of patients in Alabama and elsewhere that may require hip revision surgery in the future.
Injured? Contact us Today
Those that may have been adversely affected by a Biomet hip are encouraged to call Attorney Group for Alabama with their questions. We can help you understand your rights in your particular situation. If you decide to pursue a claim, we can connect you with an affiliated attorney who can help you file your lawsuit for compensation. Contact us today to learn more in a free consultation.