Alabama Stryker Hip Recall Attorney Warns of Alleged Stryker Hip Failure
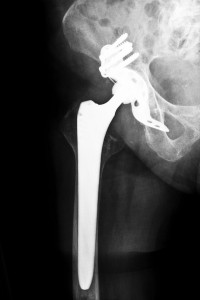
The ABG II is a Stryker hip implant that was recalled voluntarily by Stryker Orthopedics in July 2012. It was made with a certain kind of modular neck hip stem and it was determined that these hip stems displayed early rates of failure with the possible risk of corrosion and fretting while deep inside the femur bone. Many concerns are thought to be associated with this recalled device. For those who have received the Stryker ABG II replacement hip device, serious consequences are a possibility, and contacting a Alabama Stryker hip recall attorney can help determine if they have a product liability claim.
Problems Associated with ABG II
Alabama Stryker hip recall attorneys report that prior to the Stryker recall, problems were indicated for quite some time, months and even years, with the ABG II hip implant. Even though the design of this implant is different from other hip implants, not much testing was done on it before marketing was made public. The ABG II is made with four parts, the Acetabular Cup, ball, femoral stem and metal neck, whereas other hip implants are constructed with only two parts. The idea behind the new design is that more parts means better surgical options involving the angle and length for each implant. While Stryker claimed that this would improve function, it was noted by The American Association of Orthopedic Surgeons there was not any clinical evidence that function was improved by using the modular neck. The problem with the new design is that the risk of corrosion, fretting and metal ions being released increases due to the friction of metal-on-metal joints. Common reported complaints from recipients of the implant include popping or squeaking sounds from the implant, chronic pain, wearing of the implant being uneven, implant chipping or breaking, fractures and broken bones and walking difficulties. Other issues associated with this implant involve metallosis, which happens when metal rubs on metal during normal daily activities.
What to do After the Stryker Recall
Recipients of a Stryker hip implant between 2009 and July 2012 need to determine if they received this recalled hip device, the ABG II, by speaking with their physician or surgeon. Even if you are not experiencing current implant issues, you could have future problems with this device and any expenses you acquire associated with the Stryker implant should be saved for possible reimbursement. After it is discovered that you have received a Stryker ABG II hip implant, you should contact an experienced Alabama Stryker hip recall attorney to discuss your case. Attorney Group for Alabama can help you find an Alabama Stryker hip recall attorney who can protect your claim. Because product liability cases have statutes of limitations, it is very important that you have someone on your side so you do not miss any deadlines, which could forfeit your right to pursue a claim in cases like this. Once we conclude that your claim against Stryker is valid, we will pursue a claim for you and navigate you on the best course of action to fight for any compensation you are entitled to. For more information see our Stryker Hip Recall Information Center.





