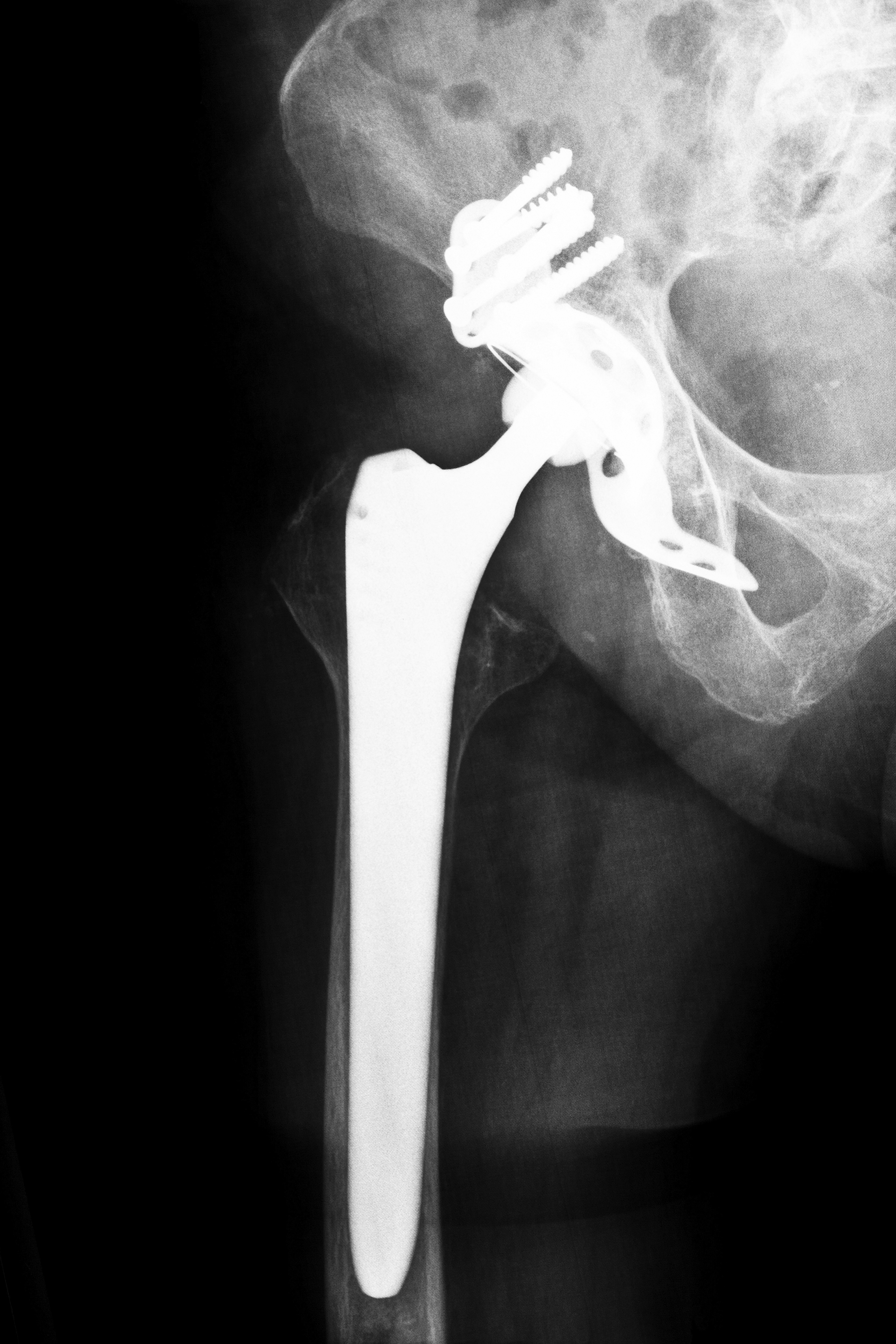
The DePuy ASR XL hip replacement device has been a source of trouble for many of its recipients, and is currently the target of a number of lawsuits. Residents of Georgia who have been implanted with the device and who are experiencing problems should be aware of the potential health issues associated with it and of their legal options.
 A product of DePuy Orthopedics, which is a division of the company Johnson & Johnson, the ASR XL Acetabular sytem was touted as one of the new generation of hip replacement devices employing all-metal components when it was introduced nearly a decade ago. Only a few years after it was approved for use by the U.S. Food and Drug Administration (FDA), many patients who had received the implant began to experience complications. Since 2008, the FDA has received more than 300 complaints about the ASR. Some of the recipients had developed aseptic lymphocyte implants vasculitis associated lesions, which are physical reactions to metal particles and ions, and pseudotumors, which are enlargements in the tissue that may also be related to foreign particulates. These health issues have necessitated the surgical replacement of the device in some patients, and it is these individuals who will require compensation to cover the additional medical costs and related expenses that will be incurred.
A product of DePuy Orthopedics, which is a division of the company Johnson & Johnson, the ASR XL Acetabular sytem was touted as one of the new generation of hip replacement devices employing all-metal components when it was introduced nearly a decade ago. Only a few years after it was approved for use by the U.S. Food and Drug Administration (FDA), many patients who had received the implant began to experience complications. Since 2008, the FDA has received more than 300 complaints about the ASR. Some of the recipients had developed aseptic lymphocyte implants vasculitis associated lesions, which are physical reactions to metal particles and ions, and pseudotumors, which are enlargements in the tissue that may also be related to foreign particulates. These health issues have necessitated the surgical replacement of the device in some patients, and it is these individuals who will require compensation to cover the additional medical costs and related expenses that will be incurred.
Initially, around 12 percent of the implants were reported to have failed, which meant that approximately one in eight recipients required corrective surgery. However, subsequent research indicated that as many as one in five recipients would need additional surgery no more than four years after the original implantation, and approximately half would need surgery after only six years. A number of orthopedists have expressed the belief that the the design of the ASR actually hampers implantation.
Two years of complaints led to the decision by the manufacturer to issue a recall of the device in the U.S. in March 2010, three months after the same type of directive was issued in Australia. The same month as the American recall, the manufacturer notified doctors of an Australian report that indicated an unusually high rate of failure in its product. Research showed that the risk of failure was the highest in patients that were of small stature, thus making failure more prevalant among women and those with weak bone structures. Even before the recall, the company announced that it planned to phase out the ASR by the end of 2010. However, this action came too late to satisfy many orthopedic experts, especially when considering that more than 90,000 of the hip devices had by that time been implanted in patients throughout the world.
The ASR was approved under a regulatory arrangement that did not require clinical trials, and according to one press account, it was marketed without adequate research and testing. This account further alleged that DePuy executives were aware of the failings in their product a year before it was formerly recalled. The reputation of the ASR was further tarnished when one of the designers of the device admitted to its deficiencies.
Since the ASR is considered a defective product, legal action for the purpose of obtaining compensation for those affected will be conducted in accordance with product liability laws. The first in a series of lawsuits related to the ASR ended in March of this year, with the plaintiff being awarded some $8 million in damages by a California state court. It has been estimated that more than 10,000 recipients of the implant could be affected by future judgments.
Those who have received ASR devices and are experiencing health problems related them should contact Attorney Group for Georgia to get in touch with an affiliated lawyer who can help them obtain the compensation that they deserve.