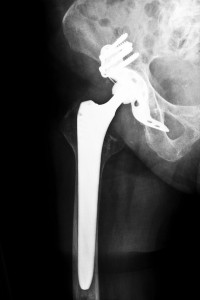
Less than 10 years ago, the manufacturers of orthopaedic hip devices were promising patients better lives, but thousands of people who had been told they would be able to walk pain free are now involved in lawsuits against the manufacturers of their devices. Faulty metal-on-metal hip replacements have been named in regards to negatively impacting the health of implant recipients, and thousands of lawsuits have been filed.
A new hip replacement that was supposedly designed to eliminate the flaws of previous models is now the subject of debate, as well as a lawsuit. The question now: Could a Kentucky Apex ARC Hip Replacement System lawsuit be next?
Massachusetts Apex ARC Lawsuit
The first lawsuit in the nation was filed in Suffolk County, Massachusetts, by a Florida woman. A resident of Fort Lauderdale is the plaintiff in the lawsuit, and Orthopaedic Synergy, Inc., the manufacturer of the Apex ARC, and its parent company, Omnilife Science, Inc., are named as the defendants.
According to the patient’s medical records, she underwent hip replacement surgery in February 2012 and had the Apex ARC device implanted into her left femur. Approximately one year later, she began to experience pain, which grew worse over time. A routine blood test showed that she had elevated levels of chromium, cobalt and titanium in her system, all of which are used to make the Apex ARC. In addition, the doctor discovered the patient had a large and chronically inflamed mass of necrotic and fibrotic tissues in her left hip near the implant, which was identified as a pseudotumor.
To correct the problems and relieve the pain, the plaintiff had to undergo hip revision surgery, sciatic nerve neurolysis and a procedure to debulk the pseudotumor. As the doctor was performing these procedures, the taper junction of the Apex ARC was found to be corroded.
Should You Consider a Kentucky Apex ARC Hip Replacement System Lawsuit?
One of the reasons why attorneys believe that a Kentucky Apex ARC Hip Replacement System lawsuit should be considered for anyone with problems similar to this plaintiff’s is that Omnilife Science marketed the device as a safe alternative to metal-on-metal hip implants, and it has become a possibility that that is not the case.
Although Omnilife Science claims that the Apex ARC is unique among hip implants, photos and diagrams show that it is strikingly similar to the Rejuvenate device that was recalled by Stryker after thousands of lawsuits were filed and complaints registered with the U.S. Food and Drug Administration (FDA).
Contact Attorney Group for Kentucky Today
Anyone living in Kentucky who has had a hip replacement and later suffered injury or had to undergo hip revision surgery should consider contacting an experienced attorney to discuss the terms of a Kentucky Apex ARC Hip Replacement System lawsuit. At Attorney Group for Kentucky, we want to help you understand your options. If you decide to pursue a claim, we can also connect you with an affiliated attorney who can assist you in filing a lawsuit. Contact us today for your free consultation





