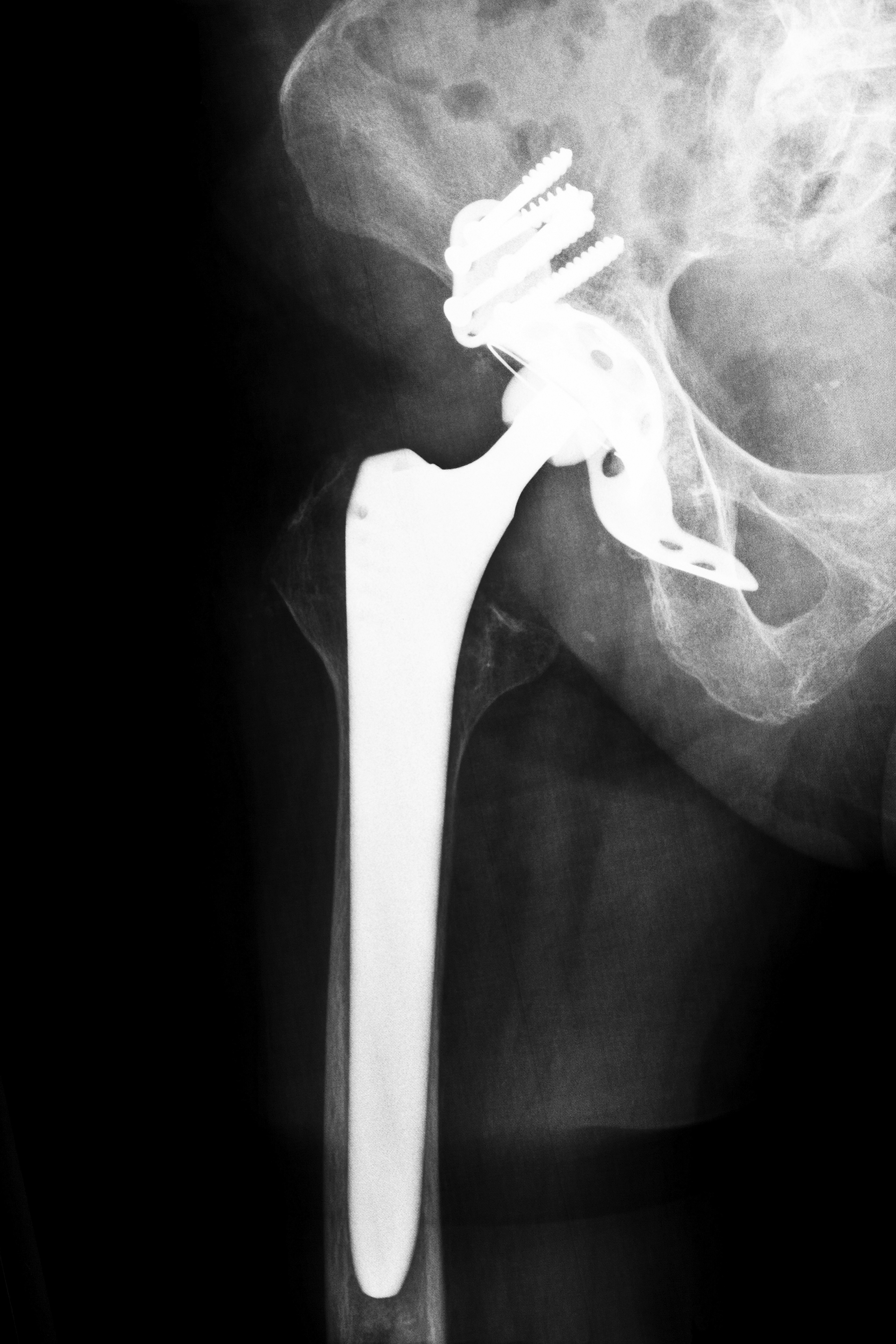
Stryker Rejuvenate Hip Recalls in Louisiana
If you have a recalled hip and would like immediate information about the Louisiana Stryker Hip Recall 2012, call now or fill out our contact form. To learn more before calling, read on. Released in 2009, Stryker Rejuvenate hip replacement systems are made from several neck and stem interchangeable components. The purpose of this design was to give surgeons flexibility when implanting the device. Patients could receive a custom-fitted implant from an anatomically correct implant. While this appeared to be an innovative solution for hip implant surgeries, repeated reports by patients of problems has raised serious concerns with the Stryker system.
Like most hip implant devices, the Stryker hip replacement system involves a metal-on-metal design. Rubbing of the metal components causes tiny particles of chromium and cobalt to enter a patient’s blood stream or surrounding tissues. Irritation, pain and other serious complications can occur.
If you live in the state of Louisiana and received one of these hip replacement devices, you may wish to consult with Louisiana Stryker hip recall lawyers. You could be at risk for the dangerous side effects associated with Metallosis, which is metal poisoning. Attorney Group for Louisiana can help you navigate the legal challenges if you have any of the following complications:
• Hip device wear and tear
• Metallosis
• Broken hip device
• Pseudo tumors
• Allergic reaction or hypersensitivity to the implant
• Swelling or pain in the hip, groin or leg that is unexplainable
Louisiana Stryker Hip Recall 2012 – Health Risks Associated with the Stryker Rejuvenate Hip Replacement System
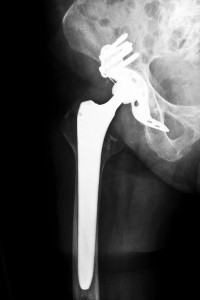 The Stryker Rejuvenate hip replacement system has not escaped the high failure rates plaguing metal-on-metal implant. These devices are capable of deteriorating over time and creating metal debris. As a result, metallic molecules of chromium and cobalt are released into the body, which can cause an autoimmune response. You may also have further complications from an allergic reaction to the metal.
The Stryker Rejuvenate hip replacement system has not escaped the high failure rates plaguing metal-on-metal implant. These devices are capable of deteriorating over time and creating metal debris. As a result, metallic molecules of chromium and cobalt are released into the body, which can cause an autoimmune response. You may also have further complications from an allergic reaction to the metal.
Other health risks associated with the Stryker hip implant may include:
• Problems with the nervous system
• Hip joint failure
• Gastrointestinal problems
• Limited mobility in the hip
• Headaches
• Dizziness
• Emotional disturbances
• Recurring problems with infections
• Bone dissolution
You should contact your physician if you have any of the above listed conditions after receiving a Stryker hip implant. Follow that call with one to an attorney who can provide legal counsel regarding your rights to receive compensation.
Voluntary Recall by the Manufacturer of Stryker Hip Systems | Louisiana Stryker Hip Recall 2012
Typically, most hip implant devices are made with a one-piece neck and stem. The Stryker Rejuvenate system is different with six stems and 16 necks, and promises of longer-lasting positive results for patients. Coated titanium stems with cobalt and chromium necks, the Stryker system causes many of the same complications associated with other metal-on-metal hip devices.
The Louisiana Stryker Hip Recall 2012
In April 2012, Stryker issued a warning to surgeons of potential hazards with its devices. This was followed by a voluntary recall in June due to the manifestation of medical problems. Two Stryker hip replacement systems were part of the Louisiana Stryker Hip Recall 2012 voluntary recall: Rejuvenate Modular Hip System and ABG II Modular-Neck Hip Stem
As a patient in Louisiana, you can verify which model was implanted from the operating report at the hospital where your surgeon performed the implant. You may also contact your surgeon directly if you are unsure of which hip implant device he or she used. If you experience any difficulty in verifying this information, one of our affiliate attorneys can assist you.
Options for Treatment
The options for treatment after receiving a Stryker hip implant are somewhat limited. Many people have repeated revision surgeries to replace the failed devices. Even minor surgeries are risky; undergoing a complicated procedure like revision surgery is potentially dangerous. Despite the inherent risks, you usually have no other choice to regain normal function of your hip mobility.
Some surgeons believe that revision surgery is difficult to perform and could lead to a longer recovery. The surgery involves dislocating your hip before problematic parts from the device are removed. Next, the cup-shaped cavity is cleaned and lined with bone graft. This is challenging to not disturb the femur while performing an additional bone graft to correct the problem.
Know Your Legal Options
Currently, Stryker is offering to reimburse affected patients for revision surgery and other expenses related to the failure of its device. The company is working with an administrator who handles third-party claims for reimbursement. In addition to surgery reimbursement, you may have the right to receive compensation for:
• Mental, physical and emotional pain and suffering
• Loss of current and future income
• Loss of consortium
• Punitive damages
Contact us about the Louisiana Stryker Hip Recall 2012 Today
Our Attorney Group for Louisiana attorneys have experience with the recent Louisiana Stryker Hip Recall 2012. We offer a free consultation session for affected patients throughout Louisiana. If you or someone you love underwent a Stryker Rejuvenate hip implant surgery and have experienced serious side effects such as hip dislocation, revision surgery or metal poisoning, you might be entitled to monetary compensation. Call us today so that we can evaluate your claim and explain the legal options available to you.