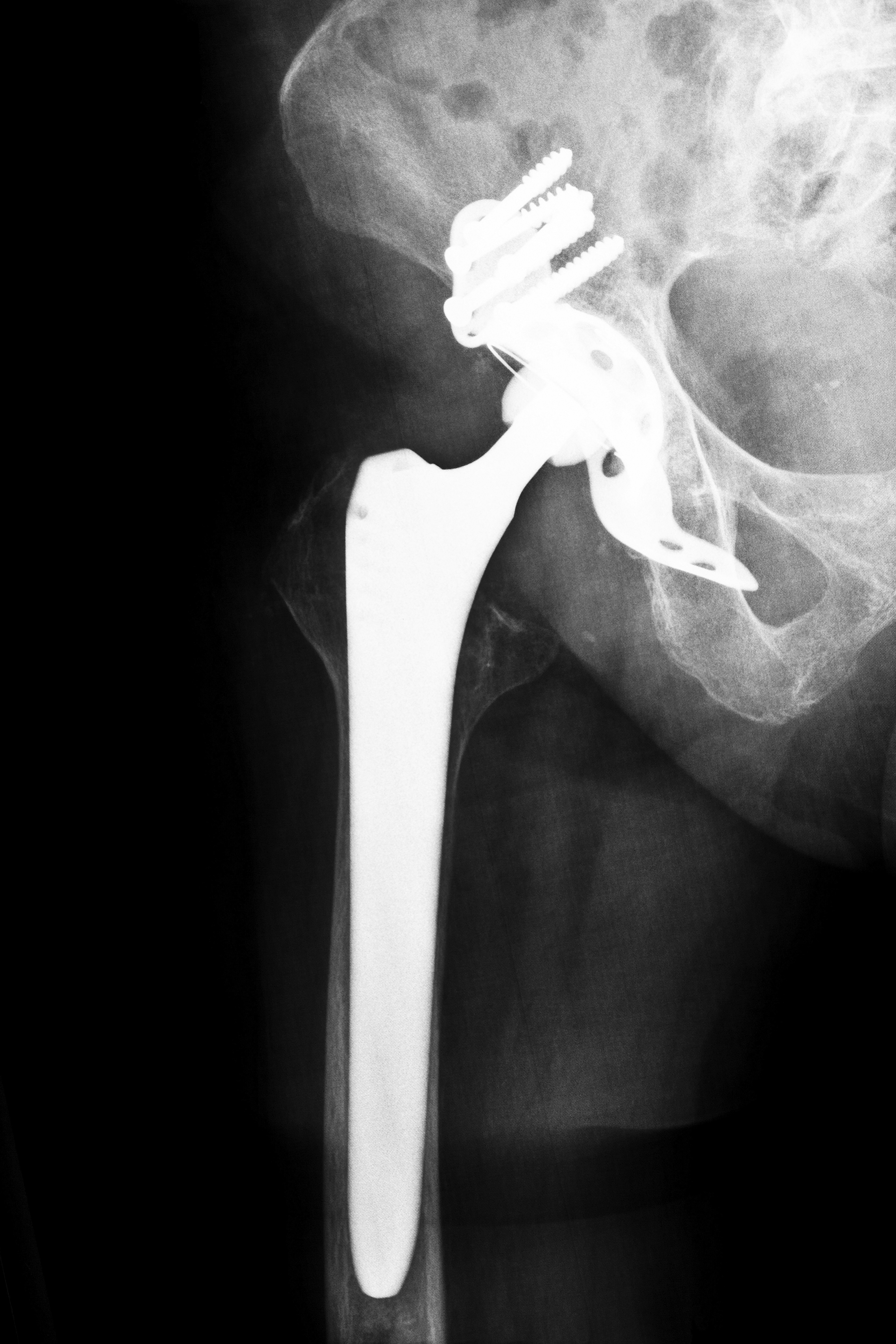
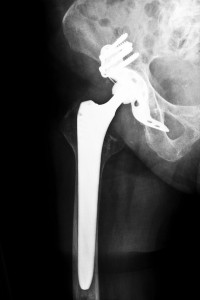
 Several manufacturers of hip implants are involved in litigation due to complications allegedly caused by their devices. Patients who believed they were getting a better quality of life claim that instead they are left with life altering and sometimes life threatening conditions. Many have also had to undergo revision surgeries with additional risks and complications.
Several manufacturers of hip implants are involved in litigation due to complications allegedly caused by their devices. Patients who believed they were getting a better quality of life claim that instead they are left with life altering and sometimes life threatening conditions. Many have also had to undergo revision surgeries with additional risks and complications.
Johnson and Johnson, the parent company of DePuy Orthopedics, has set aside $4 billion to settle hip implant lawsuits over the Pinnacle and ASR devices. The first of 6,000 Pinnacle lawsuits, filed with the federal court in Dallas, went to trial in September 2014. The lawsuit alleges that the implant released cobalt into the plaintiff’s bloodstream, causing those levels to rise to 85 times the normal level. Attorneys in the case cite documents that Dr. Thomas Schmalzried, a surgeon in Los Angeles and a consultant for DePuy, approached the manufacturer with concerns about product safety in 2001. Metal on metal hip replacement concerns were highlighted in August of 2010, when DePuy recalled 93,000 ASR hip replacements. Over 7,000 lawsuits were settled over that device for $2.5 billion in 2013.
Hundreds of lawsuits have been filed in federal court, and many more have been filed in state courts around the country against Stryker Orthopedics, the maker of the Rejuvenate and AB II Modular-Neck Hip Stem. The number of hip implant lawsuits continues to rise in a multicounty litigation in New Jersey’s Bergen County Superior Court. As of September 10th of 2014, 2,097 cases were pending in the proceeding. Federal multidistrict litigation is also underway in Minnesota with just over 1,700 cases filed.
Smith and Nephew, maker of the R3 Acetabular, is facing lawsuits filed by those who allegedly have suffered early failure of the device, metal poisoning, and other complications. The orthopedic manufacturer settled a recent lawsuit for $11.3 million, over allegations that it sold devices to the U.S. government that it claimed were manufactured in the U.S, but were instead made in Malaysia.
As of May 15, 2014, at least, 2,210 Biomet hip replacement lawsuits have been filed in a federal multidistrict litigation in the U.S. District Court in the Northern District of Indiana. Individuals in the lawsuits suffered metallosis, pseudotumor formation, chronic pain, and other complications allegedly because of the M2A hip system manufactured by Biomet.
Hip implant lawsuits against Wright Medical Technology, manufacturer of the Conserve hip replacement, continue to rise in multidistrict litigation in the U.S. District Court in the Northern District of Georgia. At least 397 claims were filed as of September 17, 2014. The lawsuits allege the metal on metal devices failed prematurely and caused other serious complications.
Hip implant lawsuits brought against Zimmer Holdings Inc., the maker of the Durom Cup, allege that the manufacturer made false statements about the safety and effectiveness of the device, along with a host of other claims. Zimmer is presently waiting on the European Union to approve its $13 billion dollar takeover of Biomet. The merger of the two companies will create a market giant in the musculoskeletal industry.
Considering Joining Hip Implant Lawsuits in Oklahoma?
Attorney Group for Oklahoma can help you if you are experiencing problems associated with a hip replacement or if you have undergone a revision surgery. Oklahoma residents can call Attorney Group for Oklahoma to set up a free consultation to help answer questions, determine if you have a claim, and connect you with an affiliated attorney in Oklahoma who can help you seek compensation. Contact us today to learn more.