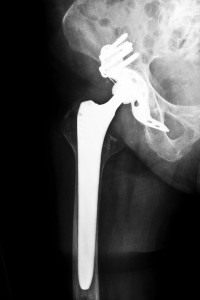
In June 2012, Smith & Nephew issued a recall for their metal liner component of the Smith & Nephew R3 Acetabular after numerous complications were reported and many reports of patients requiring additional revision surgeries. This device was introduced to the market in 2007. An estimated 7700 patients received the Smith & Nephew R3 Acetabular System. Clinical tests showed that rates for revision surgeries in recipients of the recalled R3 Acetabular System were much higher than acceptable and recipients were reporting bone fractures, dislocations and infections. Lawsuits are now being filed throughout the nation regarding the R3 metal liner component.
Metal on metal hip implants are a class of artificial hip implants that are now being reviewed by the U.S. Food & Drug Administration, FDA. When the Smith & Nephew R3 Acetabular System is used with the recalled metal liner component, it becomes an implant with metal-on-metal components. A warning was issued in January 2013 that anyone implanted with a metal-on-metal hip should get tested for excessively high metal ion levels if they suffered swelling, pain, loosening of the implant or other signs that could indicate failure of the artificial hip. The FDA has also suggested that all-metal hips be ineligible for the 510(k) clearance process, which does not make it a requirement for human clinical trials before a device is approved.
Complications with the Smith & Nephew R3 Acetabular Hip System
The R3 Acetabular metal liners, similar to the recalled DePuy and Stryker hips, are associated with abnormal failure rates and numerous complications. Metal debris is released when the metal femoral neck and metal liner grind together, causing blood toxicity and metallosis. Other common problems include increased joint pain, hip dislocations, decrease in mobility, stamina and strength, pseudotumors, bone and tissue necrosis and infections. These patients usually require corrective surgeries to remove the defective components and implant safer ones, which are complex and painful surgeries that require extensive rehabilitation.
Oklahoma Smith & Nephew R-3 Recall Attorneys
Affiliated Oklahoma Smith Nephew R-3 Recall Attorneys of Oklahoma Attorney Group are currently investigating lawsuits on behalf of recipients of a Smith & Nephew R3 Acetabular Hip System who have suffered early device failure, metal poisoning and other complications associated with this hip replacement system. If you or a loved one have experienced complications following implantation of the Smith & Nephew R3 Acetabular Hip System, contact Oklahoma Smith & Nephew R-3 Recall Attorneys today to find out if you are eligible to pursue a claim seeking monetary compensation.





