Stryker Rejuvenate Hip Replacement System Recalled
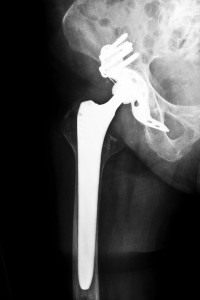
In July 2012, Stryker Orthopaedics issued a voluntary recall of its Rejuvenate as well as its ABG II modular-neck hip stems and stopped production of these devices. This was done after evidence showed that these products are prone to corrosion and fretting at the junctions of the metal modular neck, leading to breakdown and the release of toxic metal particles into the soft tissues that surround the hip, which causes swelling and pain at the joint site. In addition, this toxic metal debris can also enter the bloodstream. Oklahoma Stryker Hip Replacement Attorneys are working on product liability lawsuits to help get Stryker Rejuvenate patients compensation for they’re pain and suffering from their recalled hip implant.
What Is The Stryker Rejuvenate Implant?
Different from other metal on metal hip implants that have been the recent subject of litigation and recalls like the DePuy ASR and ASR XL hip replacement systems as well as the Zimmer Durom Cup, the Stryker Rejuvenate hip replacement system does not include a metal on metal cup and ball design. Instead, the Stryker Rejuvenate consists of a modular femoral neck. This design is different than the usual hip implant devices. Normally, hip implant devices are made with two components, a cup placed in the pelvis’ acetabulum and a femoral stem that has a ball on the end. The Stryker Rejuvenate contains four parts, a ball, a cup, a femoral stem, and a metal neck. Stryker claimed the advantages of this design would provide more options to the surgeon implanting the device. Because there are many different metal neck parts with varying lengths and angles, a surgeon has more options to choose from to fit each individual patient’s needs. However, the problem with this particular design is at the joints between the components. The necks of the Stryker hip implants are made with chromium and cobalt, and the stems have a coating of titanium. Because its components are made with metal, there is a danger of fretting with the movement of the metal joints and then corrosion of the metal neck at the femoral stem joint or the joint where the ball holds the neck segment. With activity, the friction of these metal junctions sheds metal particles that can become lodged into the nearby tissues and also enter the bloodstream.
Serious Side Effects With The Stryker Rejuvenate
Large amounts of metal debris that enter the body because of corrosion can lead to excessive inflammation, swelling and pain, pseudotumors, tenderness at the joint site, mobility decrease, hip joint loosening, toxic metal poisoning, tissue damage and death, and loss of bone. Many patients who have suffered these complications will require additional surgery to replace the implant.
Oklahoma Stryker Hip Replacement Attorneys
Even if a recipient of a Stryker Rejuvenate him implant system is not experiencing any complications, they should still see their doctor to make sure they do not have high levels of metal in their system. If you have been implanted with a Stryker Rejuvenate, contact Oklahoma Attorney Group so we can match you with our member Oklahoma Stryker Hip Replacement Attorneys. They can determine if you have a valid claim to file a lawsuit seeking monetary compensation for losses from a defective hip implant.





