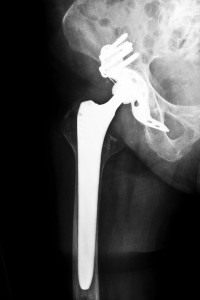
Wright Medical Technology, Inc. designed and manufactured the Wright Conserve hip replacement system, which is a metal-on-metal hip implant replacement system that was approved by the U.S. Food and Drug Administration through a quickened 510(k) process, which only requires the manufacturer to demonstrate that the medical device is substantially equivalent to other devices already on the market. By seeking FDA approval by the U.S. Food and Drug Administration through the 510(k) process, Wright Medical Technology, Inc. did not have to do pre-market safety tests. Since it was approved for market by the FDA, over 200 reports have been submitted for adverse events. Additionally, patients who were implanted with these Wright metal-on-metal hip replacement systems have filed lawsuits claiming that the devices were designed with defects, leading to early failure. Oklahoma Wright Conserve Hip Recall Attorneys are working with many patients with defective hip implants with their lawsuits
Design Defects Of The Wright Metal-On-Metal Hip Replacement System
The Wright metal-on-metal hip implant design involves a metal ball that fits inside a metal acetabular cup. Both the cup and the ball are made of cobalt and chromium. The Wright Conserve hip replacement system has striking similarities to the ASR hip implants, which have been recalled, such as neither the Wright Conserve nor the DePuy ASR hip implant systems use a liner. Thus, when the metal femoral head moves within the metal cup, these metal components grind together with each other, which causes metal particles to shed. This accumulation of metal particles can cause damage to surrounding tissues and can also enter the bloodstream. Among other complications, recipients of the Wright metal-on-metal hip implant may also suffer from dealing with the following serious side effects:
- metallosis from metal toxicity
- infections
- necrosis of tissues
- pseudotumors
- inflammation
- early hip replacement failure
These dangerous side effects can lead to premature hip replacement failure, requiring patients to undergo additional revision surgery to fix or replace the defective implant. In addition to hip implant failure, new studies suggest that metal-on-metal hips can lead to bladder and kidney cancer. Reports by the National Joint Registry for England and Wales suggest that about one in 12 recipients of the Wright Conserve hip implant will need to have a revision surgery within five years of the original implantation.
Contact An Oklahoma Wright Conserve Hip Recall Attorney
If you or a loved one has received a Wright Conserve hip replacement and suffered early hip replacement failure or other associated complications, you may have a valid case to file a lawsuit to seek monetary compensation for your medical bills, pain and suffering, income lost and more. Contact Oklahoma Attorney Group to speak with one of our member Oklahoma Wright Conserve Hip Recall Attorneys today.





