As the U.S. population continues to age, joint replacement surgery is becoming even more needed. For people who have lived active lifestyles, the joints such as the hips, knees, and shoulders can wear out quickly and eventually require replacement. As younger people are needing joint replacement surgeries, the longevity of artificial replacement joints, especially hips, has come under scrutiny. An estimated 700,000 joint replacement surgeries are done in the United States each year and out of those joint surgeries, 270,000 are replacements of the hips according to a survey posted in the British Medical Journal.
Originally, most hip replacements parts were made from ceramics and plastics. Due to complaints regarding wear as well as early required replacements prompted the make of metal-on-metal hip joints. Metal-on-metal hip replacement parts have been recommended for younger patients because they were thought to last longer. Some of the other benefits in design of the metal-on-metal hip devices include less loss of material due to constant rubbing with a lower risk of fracture of the device or dislocation.
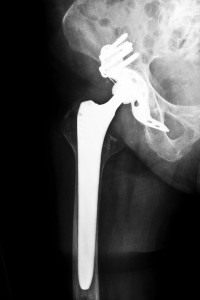
Zimmer, Inc., a manufacturer of metal hip components, made a one-piece metal socket device consisting of a plasma coating known as the Durom cup. The coating was made to stimulate bone growth onto and around the surface of the implant. The cup is kept in place then by growth of natural bone.
An estimated 12,000 people were implanted with the Zimmer Durom hip replacement from 2006 to 2008. However, due to high failure rates of these implants, sales became suspended, followed by a recall of the device in 2008. Several patients who received a Zimmer Durom Cup required another subsequent surgery to fix or replace the device. Some of these implants even failed within only several months after surgery. Other patients who received this device may have not experienced any complications yet, but still may eventually require a revision surgery in the near future.
Complications with the Durom Cup
Unfortunately though, in the hurry to introduce newly designed hip replacements for longer use, defective products were sold by several companies that have recently been under recalls. A study was published in November 2011, by the British Medical Journal comparing metal-on-metal hip implants with traditional bearings made of metal, ceramics, or polyethylene. Findings showed that metal-on-metal hip replacements do not always outlast other traditional implants. They also noted that metal debris due to the rubbing of metal parts on metal parts with movements such as walking and exercise may lead to an accumulation of metal particles in a patient’s surrounding tissues. A common problem with the Durom Cup was that the implant would not attach properly or seal with the bone. Since the implant did not need cement to hold it in place, the socket tended to float within the hip joint. Recipients complained of sharp pains in the lower back and hip. Several patients also experienced a decrease in mobility after the implantation of the Durom Cup.
Oklahoma Zimmer Durom Cup Hip Recall Attorneys
If you or a loved one are suffering from complications such as lower back or hip pain following a hip replacement surgery using the Zimmer Durom Cup, contact Oklahoma Attorney Group. Our member Oklahoma Zimmer Durom Cup Hip Recall Attorneys will protect your legal rights and ensure you receive financial compensation for your injuries.





