Have you received a DePuy ASR hip implant? Thousands of patients across the United States received the DePuy Orthopaedics defective ASR hip implant system from 2005 to 2010, when it was then recalled. Many of those recipients experienced premature failure of their DePuy ASR hip replacement system within only a few years of their surgery. Even patients who have not yet experienced complications with their DePuy ASR hip replacement face an uncertain future.
The DePuy ASR Acetabular Cup Hip Implant System was first put on the market in 2005. The U.S. Food & Drug Administration cleared this device through a process known as the 510(k) approval, which is a process that allows a device manufacturer to gain market approval without much clinical testing of the device, to include no testing on humans, if it can be proven by the manufacturer that it is “substantially similar” to another product that is already on the market. Ironically, in 2010, an internal U.S. Food & Drug Administration review was released shortly after the recall of the DePuy ASR hip implant because numerous flaws with the 510(k) process were found, which prompted the agency to think about changing it.
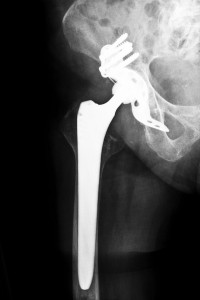
The DePuy ASR hip replacement system is a metal-on-metal device that is made of cobalt and chromium and contains a cup that is implanted in the hip with a ball joint connecting to the leg. Out of the estimated 250,000 hip replacements done yearly, about one-third of those hip replacements are metal-on-metal hip implants. However, several of the leading orthopedic surgeons have either decreased or stopped using these devices due to concerns that they are causing severe bone and tissue damage due to metal toxicity from wearing of the metal components.
The most common ways that the DePuy ASR hip implant have been found to fail involve loosening of various implant components, soft tissue damage or necrosis due to accumulation of metal debris from wearing of the implant, and elevated levels of chromium and cobalt in the blood caused by wearing of the implant. Common symptoms DePuy ASR hip implant recipients are suffering from include severe pain around the implant area and into the groin or back, destruction of cells or osteolysis, collections of fluid and solid or cystic masses surrounding the joint.
Texas DePuy ASR Hip Recall Attorneys
Affiliated Texas DePuy ASR Hip Recall Attorneys of Texas Attorney Group are reviewing DePuy ASR hip implant cases from recipients of this defective hip replacement system. If you had hip replacement surgery and have suffered from unexplained pain in the hip, thigh or groin area, pain when walking, pain when rising from a seated position, or pain when bearing weight, you could be the victim of a DePuy ASR hip implant that has failed. You should contact Texas Attorney Group today so we can match you with our member Texas DePuy ASR hip recall attorneys to protect your legal rights.





