Florida DePuy Hip Recalls
Johnson and Johnson’s orthopedic subsidiary DePuy Orthopaedic issued a press release in May 2013 to announce its discontinuation of two hip replacement systems, the Ultamet Metal-on-Metal Articulation and Complete Ceramic-on-Metal Acetabular systems. The decision is part of an overall strategy by the company to simplify its product lines. DePuy emphasized that these products were being discontinued and were not part of Florida DePuy hip recalls. The products were discontinued because they were not widely used in surgical procedures and represented only a small percentage of the company’s overall sales.
New Systems Discontinued in August 2013
Most orthopedic surgeons use hip replacement systems that are ceramic-on-polyethylene, ceramic-on-ceramic and metal-on-polyethylene. Metal-on-metal systems made up about 20% of hip replacements in 2007, but by 2012 less than 2% of new hip replacements used metal-on-metal systems. These types of systems only made up about one percent of all of DePuy’s sales. The ceramic-on-metal system was never widely used. The Ultamet and Complete systems will no longer be available for sale after August 31, 2013. In its statement, DePuy said that more discontinuations were planned throughout 2013 and 2014.
DePuy said that the Ultamet and Complete systems had no issues with safety or failure. However, the company also said that the decision to discontinue the systems was made in response to a Food and Drug Administration announcement that metal-on-metal systems would need to pass more rigorous safety requirements. The FDA stated that it would be requiring testing of any metal-on-metal hip replacements that had not been tested in clinical trials before being marketed.
Past Florida DePuy Hip Recalls
The decision to discontinue its metal-on-metal and ceramic-on-metal hip replacement systems comes three years after massive Florida DePuy hip recalls of two other metal-on-metal hip replacement systems. Patients who received the ASR Hip Resurfacing System and the ASR XL Acetabular System needed revision surgeries because of pain and other side effects related to system failures. The Florida DePuy hip recalls affected 93,000 patients.
About Hip Replacements
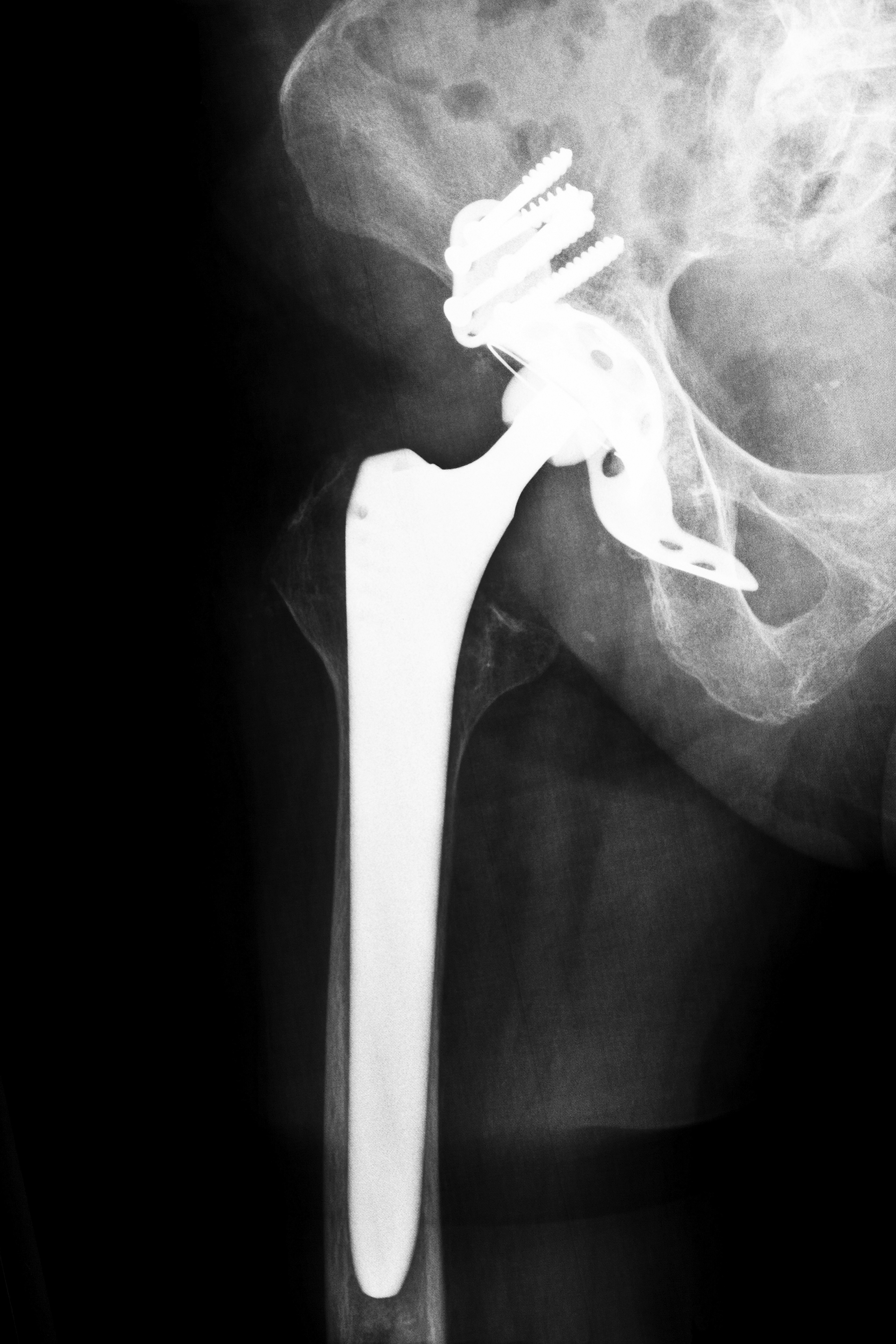
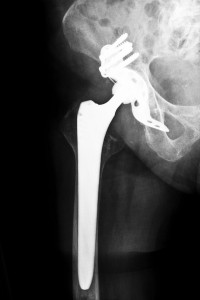 Hip replacement systems generally last from 10 to 20 years. The ASR hip replacement systems were only marketed for five years, during which thousands of patients experienced premature failures. Failures cause soft tissue damage and necrosis. Patients affected by the Florida DePuy hip recalls needed extensive additional surgeries to replace the original hip replacement and treat the damage from the ASR systems. Metal-on-metal systems like the ASR and Ultamet systems often experience wear as the materials in the artificial joint rubs together. This results in dangerously high chromium and cobalt levels in the blood. The metal fragments also damage the tissue around the joint, which caused the problems in the patients affected by the Florida DePuy hip recalls.
Hip replacement systems generally last from 10 to 20 years. The ASR hip replacement systems were only marketed for five years, during which thousands of patients experienced premature failures. Failures cause soft tissue damage and necrosis. Patients affected by the Florida DePuy hip recalls needed extensive additional surgeries to replace the original hip replacement and treat the damage from the ASR systems. Metal-on-metal systems like the ASR and Ultamet systems often experience wear as the materials in the artificial joint rubs together. This results in dangerously high chromium and cobalt levels in the blood. The metal fragments also damage the tissue around the joint, which caused the problems in the patients affected by the Florida DePuy hip recalls.
DePuy marketed the ASR products without testing the systems in clinical trials. The Food and Drug Administration allowed the company to make these systems available without first proving their safety and efficacy in a clinical setting. Nearly 11,000 lawsuits have been filed against DePuy because patients needed additional surgeries after the ASR systems were implanted. DePuy announced it would discontinue the ASR systems just three months before it issued the recall. The company’s prior history may indicate that the Ultamet and Complete system may be subject to more Florida DePuy hip recalls.
Contact Our Office Today
Attorney Group for Florida helped many people affected by the 2010 Florida DePuy hip recalls to get compensation from DePuy for medical bills and other expenses related to defective ASR implants. Patients affected by the previous Florida DePuy hip recalls were granted compensation for pain and suffering as well as medical bills. The firm is prepared to handle new cases for those patients negatively affected by the Florida DePuy hip recalls of the ASR I and ASR II who have yet to come forward. Contact our office today for a free, no obligation consultation.