Legal actions against the manufacturers of artificial replacement hips continue as recently, another company has been accused of developing, manufacturing, marketing and selling defective and dangerous artificial hips. The Apex ARC hip replacement system has been named in a recent lawsuit.
A Florida Apex ARC Hip Replacement System lawsuit was officially filed on June 24, 2014, for an artificial hip that was implanted in 2012. Some attorneys believe that this Florida Apex ARC Hip Replacement System lawsuit could be joined by others, and it may turn into a mass tort action. Attorney Group for Florida is able discuss options with anyone that has been injured, and connect you with an affiliated local attorney.
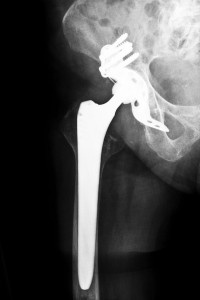
The Apex ARC Hip Replacement from Omnilife Science, Inc.
The product that is the focus of the Florida Apex ARC Hip Replacement System lawsuit is manufactured and distributed by Orthopaedic Synergy, Inc., which is a subsidiary of Omnilife Science, Inc. According to the company’s website, the Apex ARC Hip Reconstruction Implant is a safe alternative to other implants that have been targets of previous lawsuits, including models manufactured by Stryker and Depuy. However, the Apex ARC bears a striking similarity to the Stryker Rejuvenate hip implant, and when comparing photos of the two, it appears that they were designed exactly alike.
The Apex ARC consists of a hip stem with a modular neck that accepts a bearing made from one of several different materials, including various combinations of metals, polyethylene and ceramic. Omnilife calls the design of the ARC unconventional and says that it conserves bone in the femur, which is supposedly beneficial for young, active patients. The stem is constructed of wrought cobalt chromium, and it is sprayed with titanium plasma, which is topped with a hydroxyapatite coating. The stem is available in five sizes, and the modular neck has three options, including neutral, an 8-degree angle and a 12-degree angle.
Details of the Florida Apex ARC Hip Replacement System Lawsuit
The Florida Apex ARC Hip Replacement System lawsuit was filed in Massachusetts, where Omnilife Science is headquartered, by a resident of Broward County, Florida. The hip replacement surgery took place on February 20, 2012, and the patient sought medical attention on February 20, 2013, after experiencing painful symptoms.
While examining the patient’s hip, her doctor discovered a necrotic pseudotumor related to the hip surgery. In addition, testing revealed elevated levels of cobalt, chromium and titanium in her blood and tissue. The patient underwent a surgical procedure to remove the mass that had grown in her hip, a debulking procedure to relieve pressure from veins in the area and a hip revision. Upon performing the procedures, the doctor also discovered that the taper junction of the artificial hip had corroded.
Contact Us for More Information
If you or someone close to you has experienced problems such as those detailed in the Florida Apex ARC Hip Replacement System lawsuit, contact Attorney Group for Florida today to discuss your legal options in a free, no-obligation consultation.





