Smith & Nephew R3 Acetabular System
In June 2012, the Smith & Nephew R3 Acetabular Hip System was recalled due to higher than expected rates of failure. Numerous reports of complications such as fractures, dislocations and infections continued to arise which led to the recall. The Food & Drug Administration, FDA, began reviewing metal on metal hip implants which prompted new suggestions for doctors to consider testing recipients of such implants for metal ions if they were experiencing signs of early hip implant failure. The FDA also proposed that new regulations be followed that would not allow metal on metal hip implant systems to be approved through the 510(k) system, which allowed certain medical devices to be put on the market without complete testing if it was shown that the device was similar to other devices already on the market. If you are a recipient of a Smith & Nephew R3 Acetabular Hip Replacement System and have experienced complications or are concerned that future complications will arise, contact Florida Attorney Group to discuss your case so we can match you with experienced Florida Smith & Nephew R-3 Recall Attorneys. Our member Florida Smith & Nephew R-3 Recall Attorneys will determine if you qualify to pursue a claim seeking compensation through a hip recall lawsuit.
Complications Associated with the Smith & Nephew R3 Acetabular Hip System
Smith & Nephew is one of the world’s largest manufacturers of medical devices, but after numerous patient complications were being reported, the recall was issued. Common side effects associated with the hip replacement system by Smith & Nephew include infections, metal poisoning (metallosis), bone fractures, pain and swelling, walking difficulties, squeaking joints, tissue necrosis, and faulty components.
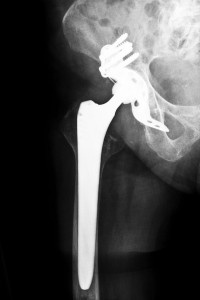
The American Academy of Orthopedic Surgeons, the AAOS, sent out a warning of the risk of a condition called metallosis, metal poisoning, associated with metal hip implants such as the Smith & Nephew R3 Acetabular Hip System. It warned patients and doctors of the possible complications including excessive pain months following surgery that could indicate metal poisoning. This can occur when metal components rub against other metal components shedding metal debris that seeps into a patient’s bloodstream and tissue. Metallosis is a dangerous condition that can cause such complications as tissue necrosis, loss of bone, inflammatory reactions, among other serious side effects.
Florida Smith & Nephew R-3 Recall Attorneys
If you have suffered complications after being implanted with the Smith & Nephew R3 Acetabular Hip Replacement System, you may qualify to receive financial compensation to help cover revision surgery expenses and pain and suffering you endured because of a defective implant. Even if you are not experiencing complications right now, you should know your rights should future complications arise since symptoms of metal ions in the blood might not always occur until later on. Our affiliated Florida Smith & Nephew R-3 Recall Attorneys will explain your legal options to you and provide an experienced team of lawyers to represent you in your defective hip implant lawsuit.





