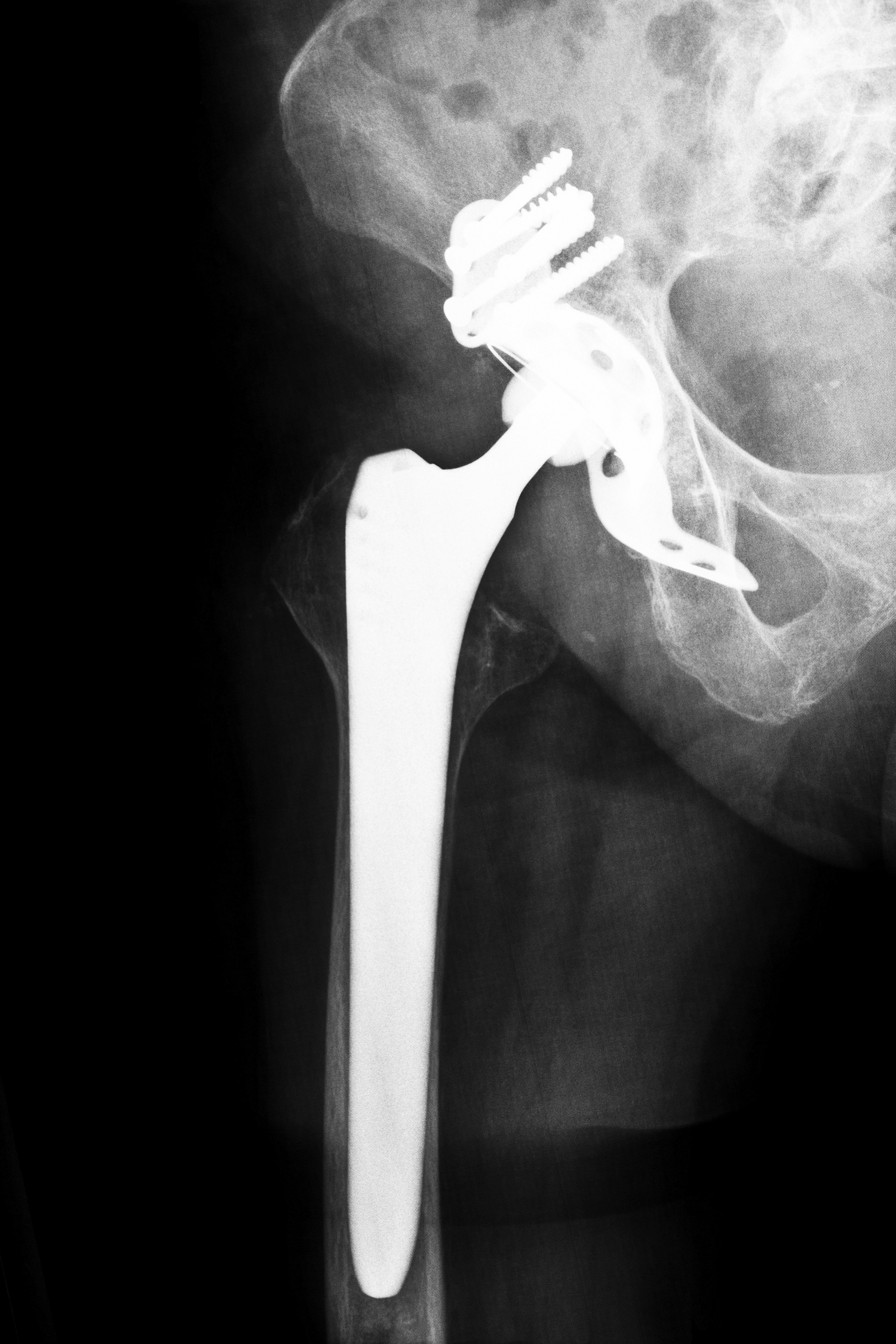
 In November 2013, DePuy officially released information on a settlement they had reached regarding hip implant lawsuits filed against them throughout the country. They were the latest in a growing number of manufacturers to take steps to settle claims of injury from patients in Kansas and other states whose hip implant reportedly failed or who suffered illness due to high levels of metal in their bloodstream. Other companies who have either offered settlements or have pending litigation include:
In November 2013, DePuy officially released information on a settlement they had reached regarding hip implant lawsuits filed against them throughout the country. They were the latest in a growing number of manufacturers to take steps to settle claims of injury from patients in Kansas and other states whose hip implant reportedly failed or who suffered illness due to high levels of metal in their bloodstream. Other companies who have either offered settlements or have pending litigation include:
- Stryker
- Smith & Nephew
- Biomet
- Wright Conserve
- Zimmer
According to the Kansas City Legal Examiner, more than 12,000 patients have filed hip implant lawsuits in state and federal courts throughout the United States. According to court documents, the hip implant’s metal-on-metal design led to a high failure rate and allowed toxic metals to enter the body as they flaked from the device during normal movement. Internal documents from many of the manufacturers that were used in court stated that the company was aware that patients would need revision surgery within five years. Once that information was known, the companies still did not recall the devices. Instead, in 2009, they began to “phase out,” but still sell, the devices. It was not until 2010 that they began recalling the defective hip implants.
In December 2013, the Kansas City Legal Examiner reported that Stryker Corporation said that they could spend between $700 million and $1.13 billion to resolve hip implant lawsuits filed against them by patients who suffered after the company’s Rejuvenate and Stryker ABG II hip implant systems were used in their hip replacement surgery. The Rejuvenate, which was designed to replace metal-on-metal hip implants has ceramic components, parts of the device have metal-on-metal interaction. This has led metal debris to flake from the device and enter the bloodstream of patients, causing a condition known as metallosis. In addition, the devices were found to have a high failure rate, leading patients in Kansas and other states to seek hip implant lawsuits for pain, swelling, pseudotumors, necrosis and bone damage that occurred after their hip surgery.
According to a New York Times article in 2011, some patients suffered significant injury from the hip replacement devices. In one case, doctors found matted strands of tissue stained gray and black as well as a large strip of muscle near the hip that no longer contracted. A New England Journal of Medicine study found that metal-on-metal hip implants failed at three times the rate of other artificial hips.
The United States Food and Drug Administration (FDA), which is designed to protect consumers from faulty medical devices, determined that there were problems with the hips and ordered an in-depth study of metal-on-metal systems. However, according to the Kansas City Legal Examiner, the FDA left it up to manufacturers how the studies were to be conducted. The devices in question were approved by the FDA under the less stringent 501(k) program, which does not require clinical trials to document their safety.
Considering Joining Hip Implant Lawsuits?
If you or a loved one has suffered from complications related to a hip implant, contact Attorney Group for Kansas to discuss your case. We can help answer your questions in a free consultation, and connect you with an affiliated attorney if you decide to pursue a claim. Contact us today to learn more.