 What is the Apex Arc Hip System?
What is the Apex Arc Hip System?
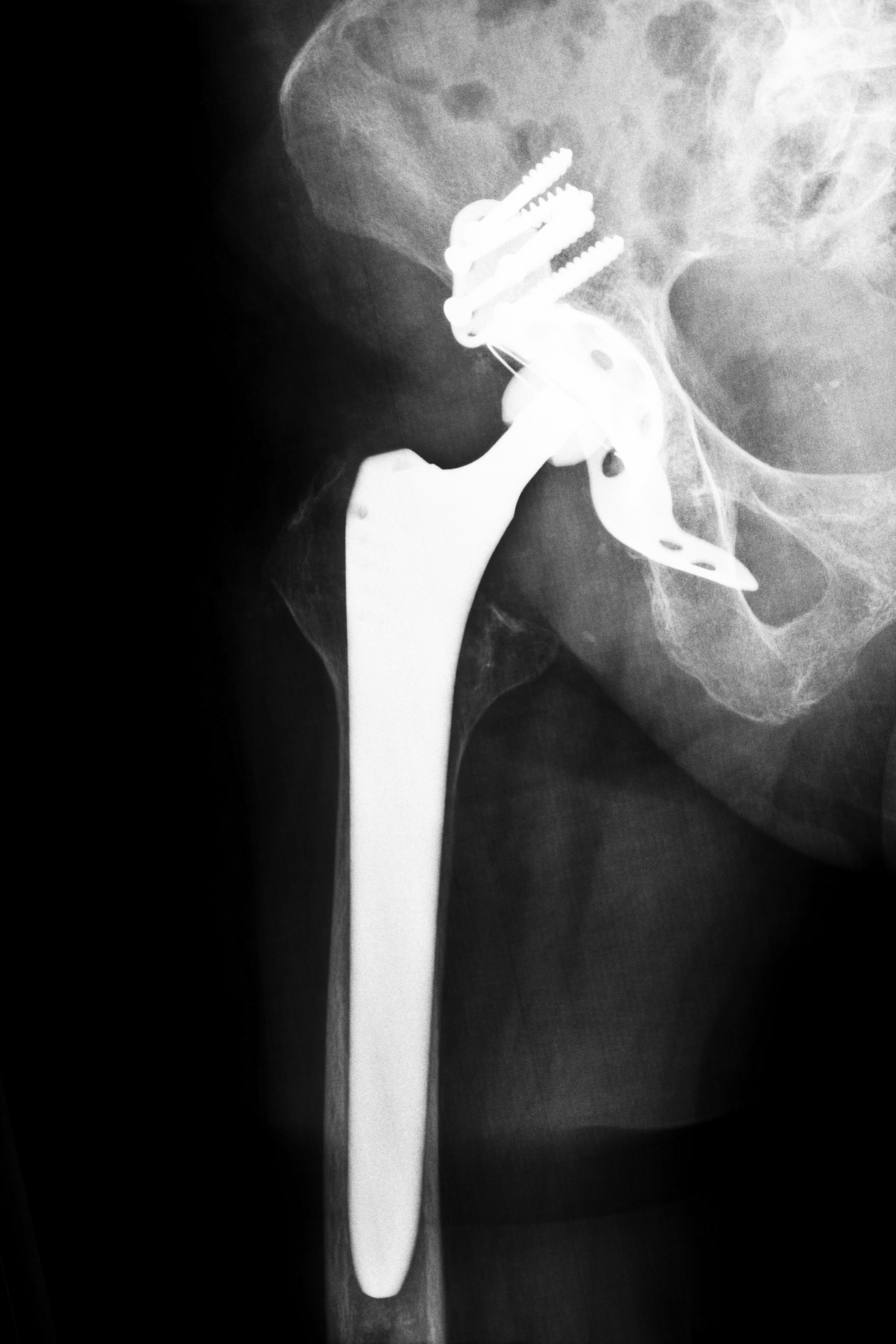
In a press release dated May 31, 2012, OmniHealth Science Inc. touted their new Apex Arc hip system as safe, bone-sparing and effective. In the same press release, Karen F. Kipfer, community relations director at Milford Hospital in Connecticut, stated that because a surgeon is given the choice of Apex Arc hip system prosthetics made with ceramic, polyethylene or a combination of metals, there is no concern of any ion release when a patient receives the OmniHealth Science implant. Many say this sounds familiar, because it’s very close to the statement made by the company that manufactured the Stryker Rejuvenate hip replacement system. They said it was safe, and then Stryker recalled the Rejuvenate prosthetic hip device fewer than 40 days after OmniHealth Science said the same thing about their similar system.
When a patient undergoes joint replacement, they should be implanted with a medical device that’s been thoroughly tested and proven to be safe. In June 2014, the first lawsuit was filed against Orthopaedic Synergy Inc. and OmniLife Science Inc. The suit, filed in OmniHealth’s home jurisdiction in Massachusetts, alleges that a Florida woman was seriously harmed by the Apex Arc hip system. The lawsuit alleges that OmniHealth was aware of defects and released the Apex Arc hip system anyway. The plaintiff had a hip replaced in 2012, and the implant began to fail within a year of surgery.
Contact Us Today to Learn More
As of June 2014, the Apex Arc hip system has not been recalled. If you or someone in your family was hurt by a hip implant, contact us. We can help answer your questions and connect you with a local affiliated attorney who can help you file your lawsuit. Contact us today for a free consultation.