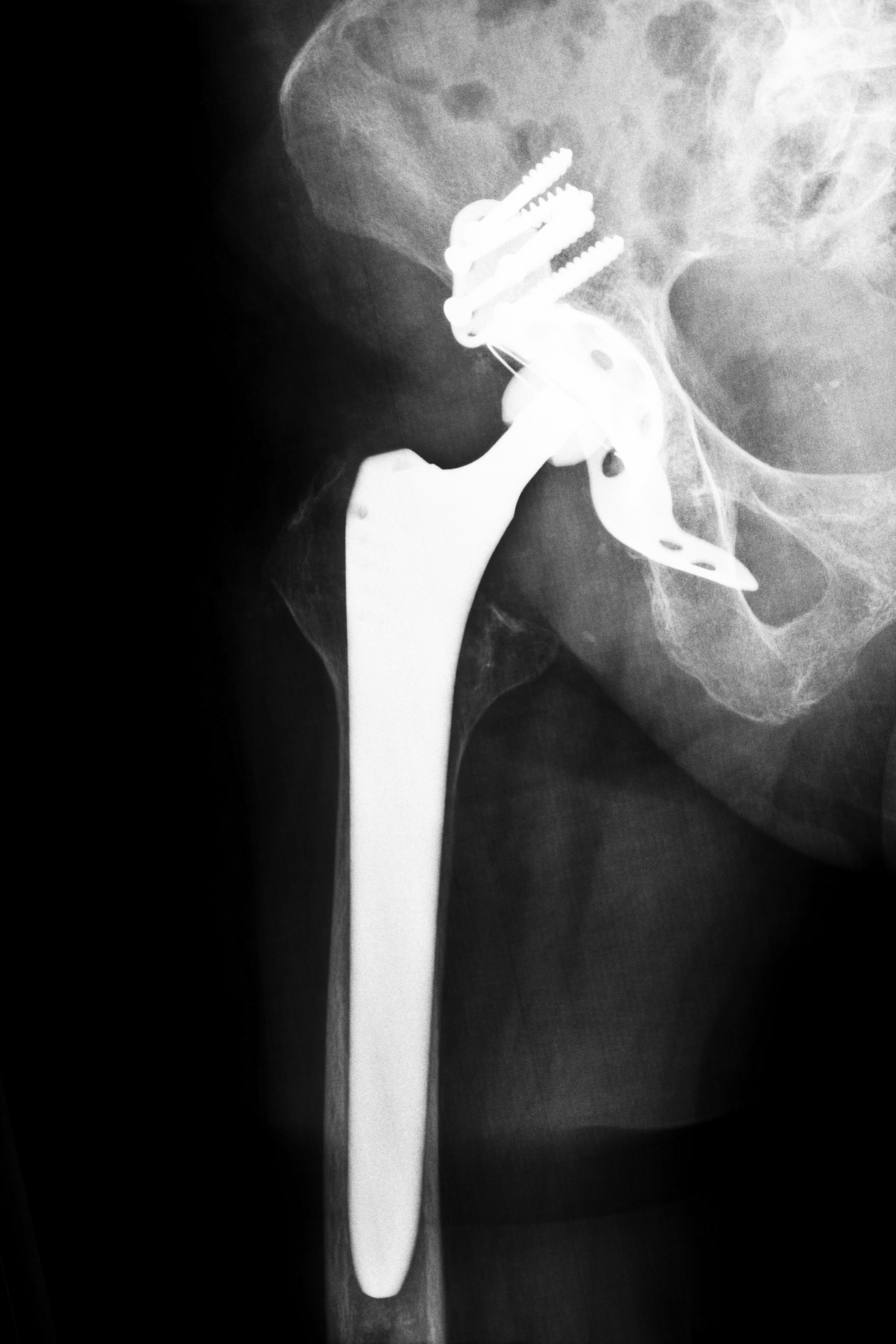
 Since 2008, there have been many metal-on-metal hip implants recalled by manufacturers, according to the United States Food and Drug Administration (FDA), and this has led to hip implant lawsuits in Kentucky and other states. Hip implants that have been recalled or that have caused complications to develop in patients over the past few years include:
Since 2008, there have been many metal-on-metal hip implants recalled by manufacturers, according to the United States Food and Drug Administration (FDA), and this has led to hip implant lawsuits in Kentucky and other states. Hip implants that have been recalled or that have caused complications to develop in patients over the past few years include:
- Depuy ASR XL Acetabular System
- Stryker Rejuvenate and ABG II
- Smith & Nephew R3 Metal Liners of the R3 Acetabular System
- Biomet M2a Magnum
- Wright Medical Technology Conserve and Profemur Z Stem
- Zimmer Durom Acetabular Component
In July 2013, a Kentucky man filed a hip replacement lawsuit against Stryker, claiming that the Rejuvenate hip implant he received in 2010 had caused him pain and discomfort before it failed soon after it was implanted. A second surgery did not resolve the patient’s medical problems, and he has suffered additional medical costs as a result of follow-up care. The Kentucky lawsuit is requesting payment for medical bills as well as punitive damages for the patient’s pain and suffering.
In February 2014, Reuters reported that Biomet had reached a $56 million settlement with hundreds of plaintiffs throughout the country. Patients in Kentucky, Illinois and many other states whose Biomet M2a 38 or M2a Magnum hip replacement systems was rectified more than 180 days after implantation would receive a base award of $200,000. In addition, a $50 million escrow account was created and a $6 million attorney fee fund established to cover all pending cases or future hip implant lawsuits filed on or before April 15, 2014. However, the company was not required to admit that its hip replacements were the cause of any injuries.
According to the FDA, metal-on-metal hip implants present unique risks to patients as the metal ball and metal cup slide against each other as the patient walks or runs. Because of the friction, metal fragments can be released and cause damage to bone or soft tissue surrounding the implant. In addition, metal fragments could enter the bloodstream and travel to other parts of the body, leading to additional illnesses or damage. A study by the National Institute of Health fond that loosening in hip with metal-on-metal hip implants are associated with hypersensitivity to metal debris. Many patients who have suffered from these complications have filed hip implant lawsuits in an effort to recover the costs of additional medical treatment, pain and suffering, as well as lost wages and other costs.
Looking to Be a Part of Kentucky Hip Implant Lawsuits?
If you or a loved one has suffered after the implantation of a hip replacement device, such as device failure, chronic pain or metal poisoning, or if a loved one has died of complications related to a hip replacement device, contact Attorney Group for Kentucky to learn the qualifications for hip implant lawsuits. We can help you understand what your options may be, and connect you with an affiliated attorney if you decide to pursue a claim. Contact us today for a free consultation.