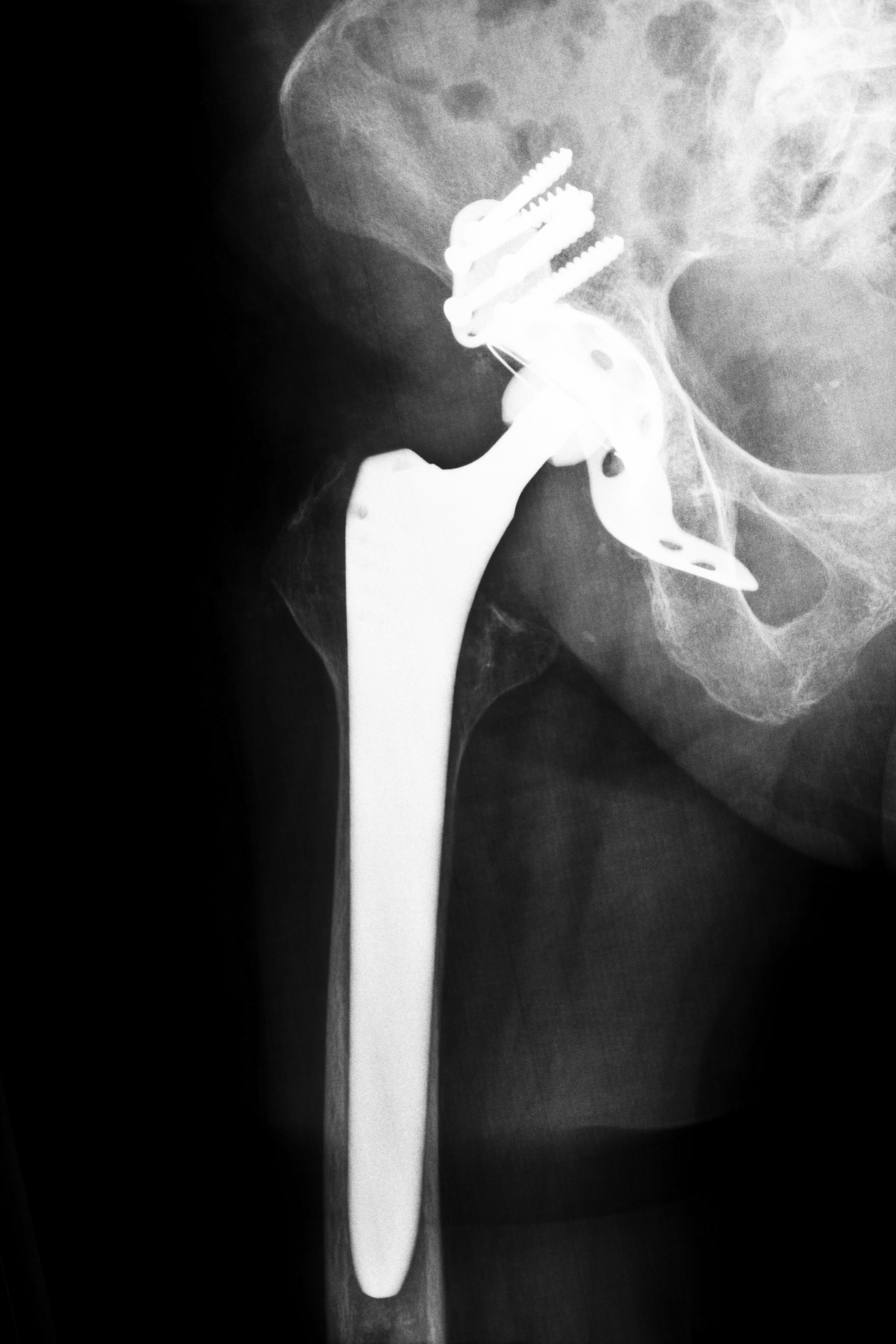
Preemptive Kentucky DePuy Hip Recalls Being Made
Johnson & Johnson announced that its subsidiary, DuPuy, plans to discontinue manufacturing of Ultamet Metal-on-Metal and Complete Ceramic-on-Metal Acetabular hip replacement systems at the end of August, claiming they are simply streamlining their products. The company denied that these were part of any Kentucky DePuy hip recalls and had nothing to do with reports of problems with the devices.
Company Statement
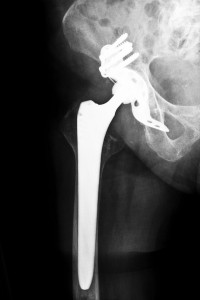 DePuy, which recalled two artificial joint systems in 2010 as part of the Kentucky DePuy hip recalls due to high failure rates, said that the Ultamet and Complete hip replacement systems represented less than one percent of their sales in the United States and Europe, as physicians now prefer a different type of bearing. In addition, the company cited new regulations proposed by the Food and Drug Administration (FDA) requiring extensive approval processes for metal-on-metal systems. The only metal-on-metal system manufactured by DuPuy is the Ultamet system. Because the Complete system is a ceramic-on-metal system, many believe that the removal of the system from the product line is actually a preemptive method to avoid Kentucky DePuy hip recalls, even though the company also stated that more products are expected to be discontinued over the next few years.
DePuy, which recalled two artificial joint systems in 2010 as part of the Kentucky DePuy hip recalls due to high failure rates, said that the Ultamet and Complete hip replacement systems represented less than one percent of their sales in the United States and Europe, as physicians now prefer a different type of bearing. In addition, the company cited new regulations proposed by the Food and Drug Administration (FDA) requiring extensive approval processes for metal-on-metal systems. The only metal-on-metal system manufactured by DuPuy is the Ultamet system. Because the Complete system is a ceramic-on-metal system, many believe that the removal of the system from the product line is actually a preemptive method to avoid Kentucky DePuy hip recalls, even though the company also stated that more products are expected to be discontinued over the next few years.
The Recalled DePuy ASR and DePuy Pinnacle Hip Systems
In these artificial hip joints a metal or ceramic ball slides against a metal cup when the patient is using the hip by walking, running, or moving. These types of artificial joints, such as the recalled DePuy ASR and DePuy Pinnacle systems (recalled due to their high failure rate), were originally designed for less wear, and a decreased chance of dislocation, which is a common problem among patients with total hip replacements. However, because the ball slides against the base, and, regardless of what the ball is created from, there is a risk of tiny particles of metal being released into the patient, and some of these metal pieces could enter the patient’s bloodstream. Should the metal enter the bloodstream, patients could develop a condition known as metallosis, which is why there is a push for full Kentucky DePuy hip recalls.
Dangers of Metal Hip Implants
Many experts believe that there are significant dangers in metal hip implants such as the recalled DePuy ASR and DePuy Pinnacle hip replacement systems, which is why many of them are calling for further Kentucky DePuy hip recalls. According to an article in Newsmax Health, research indicates that four in ten metal hip implants fail within five years. Although the failure rate is lower for ceramic-on-metal implants, the risk of metal fragments entering the bloodstream is still possible, with many doctors preferring metal-on-plastic joints. For this reason, experts are recommending Kentucky DePuy hip recalls of both metal-on-metal and ceramic-on-metal hip replacement components. In addition to the failure rates and potential for patients to develop metallosis, there have been reports of patients suffering from squeaky hip joints in those who received ceramic-on-metal components. Many of these patients needed additional surgery to eliminate the squeaking joint.
Replacement for Metal-on-Metal
Many of those calling for Kentucky DePuy hip recalls of the Complete system claim that the FDA approved the system without requiring full clinical trials, and that it was simply accepted as a replacement for many components included in previous Kentucky DePuy hip recalls. In many cases, the ceramic-on-metal hip replacement components failed, causing dislocation of the implant, bone fracture near the implant site and metallosis, increasing the need for full Kentucky DePuy hip recalls.
Learn More About Kentucky DePuy Hip Recalls
The recent announcement by Johnson & Johnson that DePuy would discontinue manufacturing of two hip replacements that have been known to cause serious health risks in patients may be a preemptive move to avoid further legal action from those have been injured by the implants. If you or a loved one have been injured due to the DePuy ASR I, ASR II, or DePuy Pinnacle hip replacement systems, or want more information about Kentucky DePuy hip recalls, contact Attorney Group for Kentucky today.