Kentucky Smith and Nephew Hip Replacement Attorneys
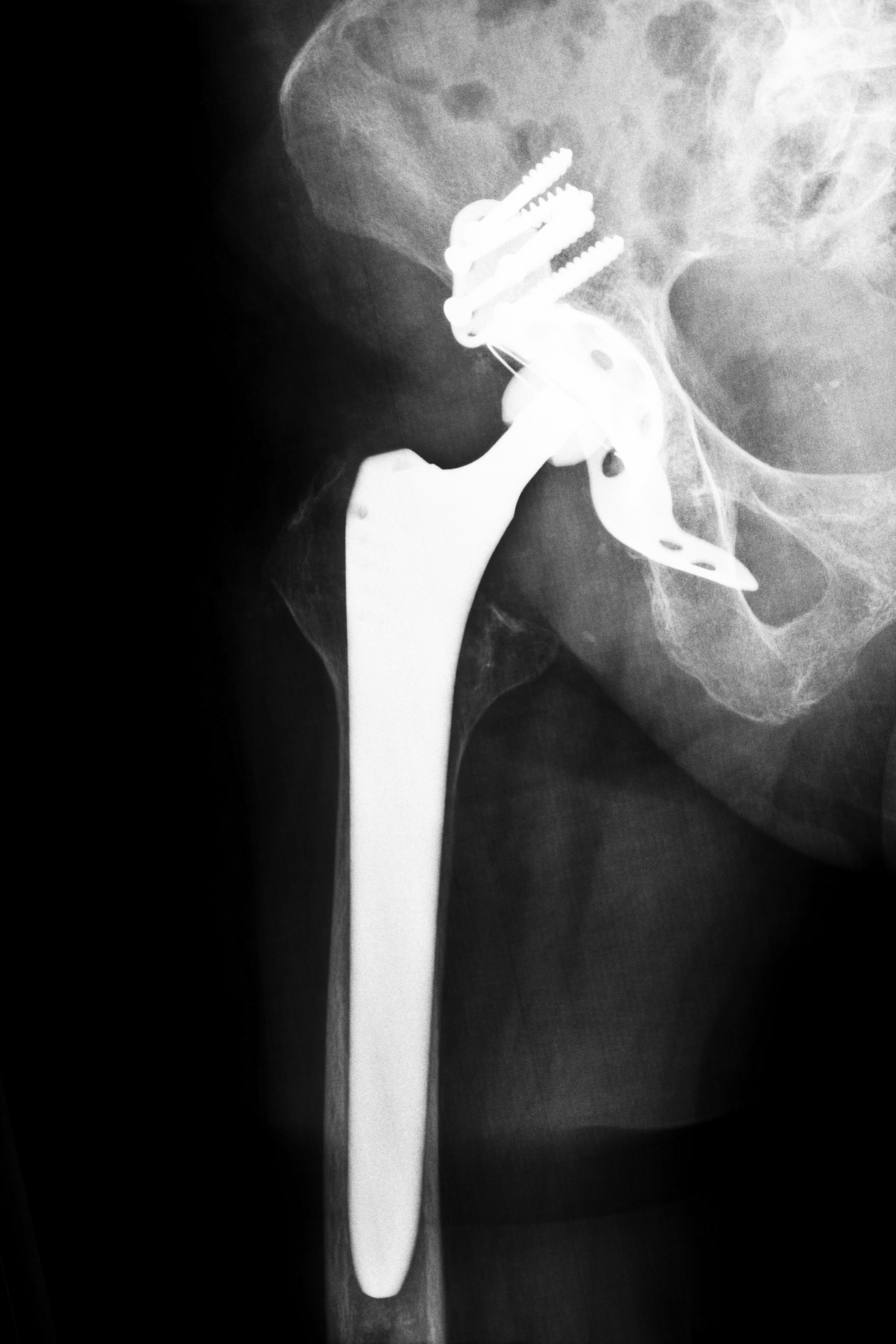
The R-3 Acetabular System was originally marketed as a device promising the widest range of advanced bearing options. The manufacturer claimed that its greater range of motion would allow for a better overall quality of life. The multi-bearing cup provides a foundation designed to reduce wear and tear, and any subsequent needs for revision surgery are decreased dramatically. However, as surgeons began to fit more patients with the device, problems became increasingly apparent. Almost 4,000 of the metal liners in the device were used in the United States between its release in 2009 and the recall issued in June 2012. If you have suffered from a defective system, contact Kentucky Smith and Nephew Hip Replacement Attorneys to learn about your rights.
Brief History of the R-3
Smith and Nephew, a company that is based out of London, has successfully manufactured several lines of replacement systems for shoulders, knees, and hips. The company is America’s fourth biggest manufacturer of prosthetic hips. The R-3 Acetabular System was originally released in Australia and Europe in 2007; it was not until 2009 that the device was marketed in the United States. On June 1, 2012, the manufacturer issued a worldwide recall of the metal liner in the device after receiving numerous reports of dislocation, metal sensitivity, infection, device failure, pain and loosening. The device demonstrated a high revision rate–6.3% over the course of four years, compared to the average rate of 2.89% for other hip replacement devices.
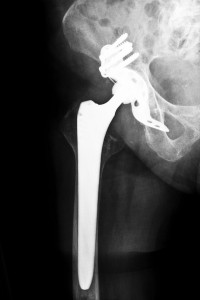
Recall of the Defective Device
According to Reuters, since its debut in 2007, nearly over 7,700 patients received the R-3 Acetabular System’s cup during surgeries. Unfortunately, many of these individuals suffered from dislocations, infections, and fractures, resulting in the manufacturer to recall the metal cup. However, because only one part was recalled, surgeons were still able to use the system for hip replacements; they merely had to substitute a non-metal liner instead.
In June 2012, Smith and Nephew issued a recall of the metal cup after they analyzed the device and found that there was a higher than average number of patient- and physician-reported problems with the device. Many individuals suffered from metallosis or metal toxicity when the metal parts rubbed together. After the recall, the company sent a letter warning doctors who had previously implanted the liners. Smith and Nephew claimed that the hazard alert was simply precautionary and that the company was unable to identify one solitary reason for the failure of the device. The letter instructed physicians to follow up with each patient that received the implant.
However, Smith and Nephew still maintains that the system is designed to give surgeons more options and enhance stability. It remains on the market and now includes cups made of ceramic, metal, or plastic. However, when the device is used with a metal cup, it becomes a metal-on-metal device. While the manufacturer marketed the metal liner as wear-resistant and durable, the device hardly lived up to such qualifiers.
Health Risks and Symptoms
Metal-on-metal hip replacement devices were originally created with the idea that they would prove to be more durable, allowing for a greater range of motion over a longer period of time. In reality, metal-on-metal hip replacement systems actually have a fail rate much higher and quicker than manufacturers originally expected, and they often require pricey corrective surgeries. Metal-on-metal devices are often the cause of many dangerous, painful side effects including metal toxicity, bone deterioration, dislocation, loosening of the implant, severe pain with or without movement, swelling and inflammation around or directly at the site of implantation, and additional revision surgeries. These serious side effects are leading many to consult with Kentucky Smith and Nephew Hip Replacement Attorneys to learn more about their rights.
In addition, metal-on-metal implants also have a greater risk of causing metallosis, or metal toxicity, stemming from the friction that occurs when the metal parts rub against one another and release metal ions into the body. As the metal pieces scrape together, not only do they weaken the implant itself, but flakes of metal frequently accumulate around the area and often end up in the bloodstream. Metallosis can be known to cause additional serious side effects including infections, emotional issues, confusions, gastrointestinal complications, dizziness and headaches. Moreover, patients have reported complications with their nervous systems such as numbness, tingling and burning in their arms and legs. Some patients may also suffer from cobalt poisoning and may likely experience sight, cognition or hearing issues, tremors, cardiomyopathy, hypothyroidism and skin rashes. It is because of side-effects like these that patients turn to Kentucky Smith and Nephew Hip Replacement Attorneys to be compensate for their pain.
How You Can Seek Help
If you or someone you love underwent surgery for implantation of the Smith and Nephew R3 hip replacement device, you may be eligible to receive compensation. Contact Kentucky Smith and Nephew Hip Replacement Attorneys who can help you to determine if you can file for damages including pain and suffering, future costs for revision surgery, any loss of earnings or compensation for your past, present and future medical bills. Oftentimes, there are time constraints in similar cases because of the statutes of limitations that vary from state to state, so if you believe that you have a case, contact Attorney Group for Kentucky for a free, no-obligation consultation today.