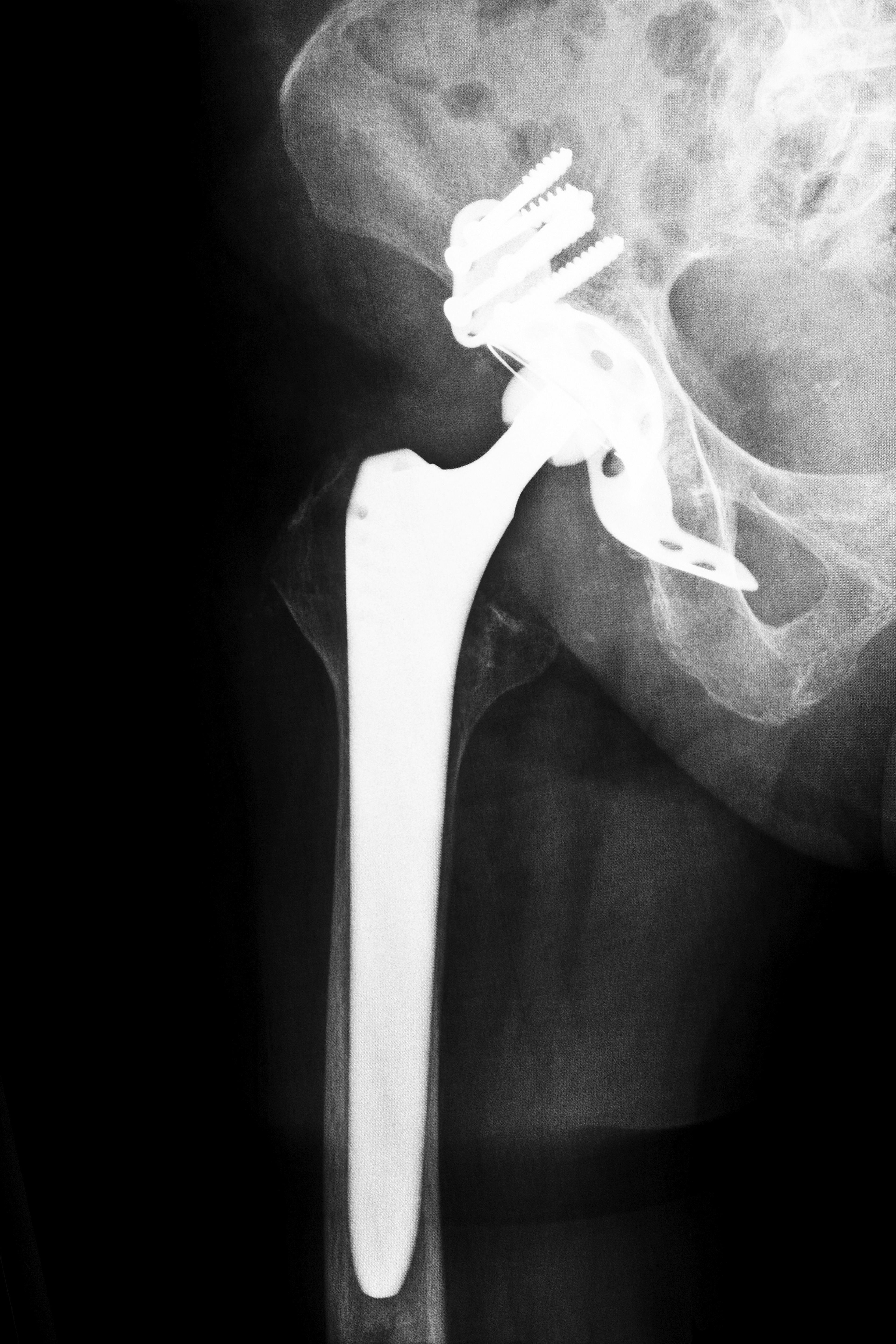
 Thousands of lawsuits have been filed against Stryker Corporation of New Jersey and Depuy, a subsidiary of Johnson & Johnson, after the companies’ hip replacement systems caused injuries and chronic pain for many. It appears now that Stryker and Depuy are not the only companies producing metal-on-metal hip replacements that could potentially fail.
Thousands of lawsuits have been filed against Stryker Corporation of New Jersey and Depuy, a subsidiary of Johnson & Johnson, after the companies’ hip replacement systems caused injuries and chronic pain for many. It appears now that Stryker and Depuy are not the only companies producing metal-on-metal hip replacements that could potentially fail.
OmniLife Science, manufacturer of the Apex Arc Hip System, was named in a recent lawsuits filed by a Florida woman in Massachusetts. The lawsuit, as well as thousands of other complaints that have been filed with the FDA, claim patients have been experiencing one or more of these symptoms:
- Corrosion: Both companies have received complaints that the hip implant deteriorates due to the grinding of metal components.
- Metal Poisoning: Metal-on-metal friction can product debris that over time causes a condition known as metallosis.
- Weakening Bones: Bones that surround the hip implant are prone to weakening, otherwise known as osteolysis.
- Displacement: Stryker implants oftentimes became displaced. A patient is then forced to undergo a second procedure to correct the problem.
- Dislocation: Some patients complained that the hip replacement device separated into two parts, causing pain and forcing them to endure a second surgery.
- Broken Bones: Many patients suffered from bone fractures in the area of the implant.
Stryker, Johnson & Johnson and OmniLife Science have all received similar complaints about their hip replacement systems over the past few years. Both Stryker and Johnson & Johnson agreed to settle certain claims at the end of last year. Johnson & Johnson settled 8,000 lawsuits with a $2.5 billion dollar settlement. It has been reported that Stryker is settling claims for up to $50K per patient. Lawsuits from Texas and other states across the country started shortly after the two companies issued voluntary recalls back in 2012.
Patients with the Apex Arc Hip System should know that the metal-on-metal hip replacement system they use is made similarly to the recalled implants. When OmniLife Science went before the Food and Drug Administration (FDA) in 2010, they piggybacked on the success of the Stryker and Johnson & Johnson implants that were already approved by the administration. Apex company representatives discussed the many ways the Apex Arc Hip System was similar to their competitors’ implants in order to receive FDA approval. Most notably, all three manufacturers produced a CoCr v. Titanium modular neck and stem, according to records.
Have You Been Injured From the Apex Arc Hip System?
Many Apex Arc Hip System patients have already reported metallosis, chronic hip pain and death of bone tissue to the FDA. If you or a loved one has suffered from constant hip pain, pseudo-tumors that resulted from metal poisoning, or have had to undergo revision hip implant surgeries, contact Attorney Group for Texas. We provide a free, no-obligation consultation to help you understand your legal options and help you determine if you have a case. If you decide to pursue a claim, we can connect you with an affiliated attorney who can help you file your lawsuit. Contact us today to learn more.