Bard IVC Filter attorneys note that medical device manufacturer C.R. Bard has agreed to the settlement of a product liability claim filed on behalf of a plaintiff alleging serious injuries resulting from a Bard Recovery Inferior Vena Cava (IVC) filter. The lawsuit, filed in June 2012 in the U.S. District Court for the District of Nevada, claimed the small IVC filter broke inside the plaintiff’s body and perforated his heart. A docket entry on February 9, 2015 indicated the parties advised the Court that a settlement had been reached following six days of trial, but other details were not released.
If you or a loved one received a Bard IVC filter and experienced injuries or complications, Attorney Group can help. We offer free, no-obligation consultations and, if you have a case, we can connect you with an affiliated Bard IVC filter attorney who can assist you in pursuing your claim.
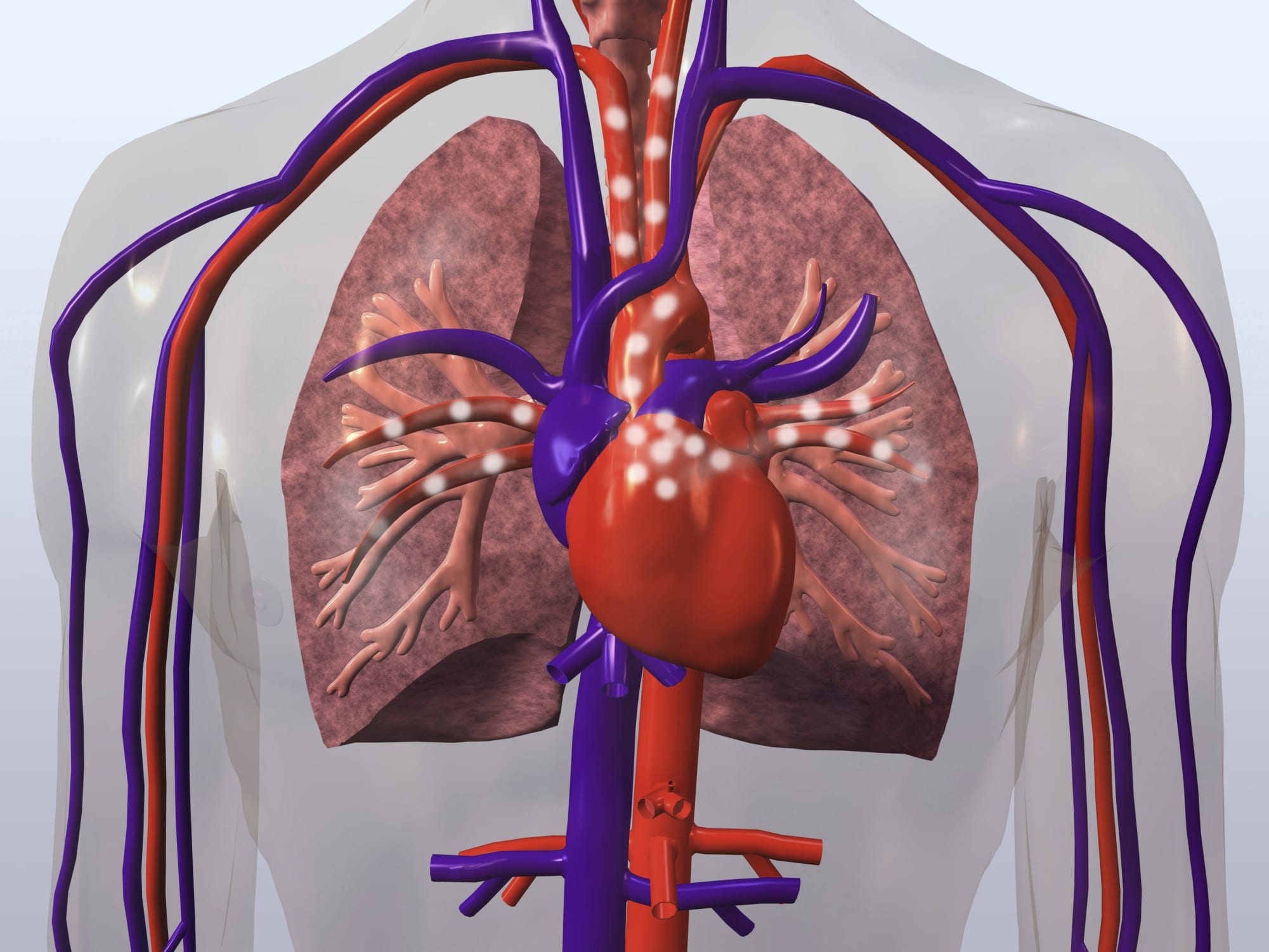
What is a Bard IVC Filter?
IVC filters are devices that are surgically implanted into the patient’s inferior vena cava vein to catch blood clots that may break free deep inside the body and enter the lungs, causing a pulmonary embolism. The filters are designed with components resembling spider legs, or struts, which expand inside the vein to prevent pulmonary embolisms.
Bard IVC Filter Lawsuit Overview
In the Nevada lawsuit, the plaintiff received a Bard Recovery IVC in 2005 and experienced complications in April 2010. At the time of the trial, the plaintiff alleged the device was inherently defective and failed to remain stable when it caught a blood clot it was designed to stop. The plaintiff claimed one of the struts broke off the device and traveled to his heart, resulting in the need for open heart surgery that required an extended, costly and painful recovery period.
The lawsuit raises allegations similar to others made against the company in recent years. Problems with the Recovery filter as well as a second-generation version called the Bard G2 IVC filter have allegedly failed in dozens of cases. In many instances, the legs either broke off the main device or allowed the filter to move to other parts of the patients’ bodies, puncturing or piercing vital organs.
The U.S. Food and Drug Administration issued an alert in August 2010 warning consumers of the potential dangers and risks associated with removal IVC filters and stated that more than 900 adverse event reports had been submitted to the agency for review. Of these, 56 involved fracturing of the filter, 70 involved perforation of the inferior vena cava, 146 involved the breaking off of components, and 328 involved the IVC filter breaking free and traveling throughout the body.
Questions About a Bard IVC Filter Lawsuit?
If you experienced complications caused by a Bard IVC filter and you would like to learn more, contact Attorney Group for a free, no-obligation case evaluation. At no out-of-pocket cost to you, we can answer your questions and connect you with an affiliated Bard IVC filter lawsuit attorney who can assist you in seeking the compensation to which you may be entitled.