A DePuy Attune lawsuit may be an option for patients who have experienced early failure of their ATTUNE Primary Total Knee System. Reports indicate that the Attune knee implant, as well as knee implants manufactured by device makers Arthrex and Exactech, may cause patients to undergo revision surgeries and experience pain and other losses. Affected patients may be eligible to seek compensation with the help of a medical device attorney.
If you have question about your DePuy Attune knee replacement or a DePuy Attune lawsuit, contact the Attorney Group today. We provide free, confidential, no-obligation consultations, and if you have a case, we can connect you with an affiliated DePuy Attune lawsuit attorney who can assist you throughout the legal process.

Have You Seen a DePuy Attune Lawsuit Commercial?
You may have seen a DePuy Attune lawsuit commercial or a commercial about lawsuits involving another knee replacement system. Numerous knee replacement lawsuits have been filed in recent years, alleging injuries to patients. The purpose of this article is to provide you with information about the issues to help you determine whether you might be eligible to file a DePuy Attune lawsuit.

What is the DePuy Attune Knee Implant?
The DePuy Synthes ATTUNE Primary Total Knee System is intended for cemented use as a total knee replacement system, for use in patients with a severely painful and/or severely disabled knee joint resulting from:
- Osteoarthritis
- Post-traumatic arthritis
- Rheumatoid arthritis, or
- A failed previous implant
According to the manufacturer, the device was designed with the goal of addressing the clinical needs of patients who require a knee replacement procedure, incorporating extensive research and science in the design to help improve functional outcomes for patients. DePuy Synthes claims that the ATTUNE Knee is an innovative, comprehensive, integrated knee system. The company also claims it is one of the largest research and development projects in the history of DePuy Synthes Joint Reconstruction combining “the latest in design, kinematics, engineering and materials to deliver stability and motion.”
What are the Risks of the DePuy Attune Synthes Attune Knee Implant?
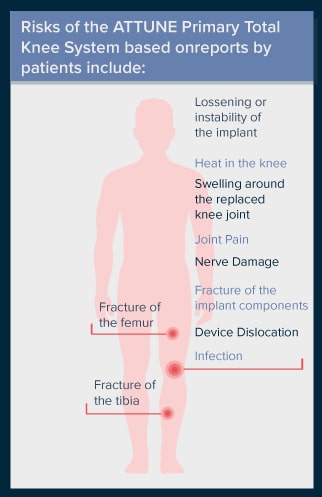
Risks of the ATTUNE Primary Total Knee System based on reports by patients include:
- Loosening or instability of the implant
- Heat in the knee
- Swelling around the replaced knee joint
- Joint pain
- Nerve damage
- Infection
- Fracture of the implant components
- Fracture of the femur or tibia
- Device dislocation
Independent studies report the ATTUNE Primary Total Knee System has experienced failures requiring revision surgeries and documented cases of death.
Has There Been a DePuy Attune Recall?
- August 2017There is not an ATTUNE® Primary Total Knee System recall. However, problems reportedly associated with the ATTUNE® Primary Total Knee System may lead to a DePuy Synthes Attune lawsuit even though no recall has been initiated. A recall of a product is not necessary for claims alleging that a product or medical device is defective and unreasonably dangerous, nor is a recall necessary for patients to recover damages for injuries allegedly caused by a defective medical device.
- June 2015The U.S. Food and Drug Administration did recall one of the instruments of the ATTUNE knee system - the Tibial Articulation Surface Trial which snaps together with the Shim component to function as the Insert Trial during the total knee replacement surgery. A Balseal, a small wire spring coil located on the post feature of the ATTUNE Knee Tibial Articulation Surface Instruments, is attached to each of the two post features on the Articulation Surface to provide a connection force between the Tibial Articulation Surface and Shim to ensure secure engagement between the components.
- November 2015The Attune FDA recall was terminated in November 2015. The FDA recall was issued after it was discovered that the small wire spring coil used in the range of motion testing to assist in tibial location prior to drilling peg holes, could become damaged and detach. This may have allowed the coil to enter the surgical site during the knee replacement surgery and remain in the patient if the surgeon was unaware that it detached.
Lauren A. on May 16, 2016
Attorney Group reviewed by:"These guys are a pleasure to work with -- very strategic and very responsive, which makes for a great business partner! I can tell that they are passionate about making sure all clients get the attention and expertise they deserve."Rating: 5 ★★★★★
DePuy Attune Knee Implant Injuries in the News
In April 2017, the National Joint Registry of England, Wales and Northern Ireland reported that between 2012 and 2016 there were 93 deaths and 46 revisions related to the with the ATTUNE knee replacement device.
Other knee replacement devices have been recalled or experienced issues in recent years.
Arthrex Knee Replacement Recall
Arthrex, a Naples, Fla., based medical device company, issued a recall of its Arthrex iBalance TKA Tibial Tray in December 2015. The recall came after patients suffered from complications requiring revision surgeries. Arthrex recalled the product after finding a smooth texture on the outer part of the knee implant. The texture made the iBalance incompatible with previous product models that had a rough texture.
(888) 888-0612
Exactech Knee Implant Failure
The FDA received reports that the Exactech Optetrak knee replacement components were failing prematurely. A study by Orthopaedics & Traumatology: Surgery & Research, showed the performance of the Exactech Optetrak in 110 prostheses in 106 patients 25 months after the knee replacement surgery.
The studies results were poor in a number of patients after receiving the implant. The knee replacement patients who were part of the study reported the following:
• 15% said they were disappointed or dissatisfied
• 22% reported pain and the need for painkillers on a regular basis
• 22% were beginning to suffer from tibial implant loosening
• 21% had signs of patellofemoral conflict
• 13 implants needed revision surgery for tibial loosening, patellofemoral instability or patellofemoral pain
The researchers noted that the tibial loosening was taking place at the cement-tibial-implant interface.
Fragmentation and wear of the polyethylene (plastic) insert were among the problems cited by researchers regarding the Exactech Optetrak knee implant.
Is There DePuy Attune Class Action?
There is no DePuy Synthes ATTUNE Primary Total Knee System class action pending as of August 2017. DePuy Attune lawsuit attorneys are doubtful that a class action will be certified for patients who are adversely affected by these knee replacements. Instead, if multiple lawsuits are filed against DePuy alleging injuries and other damages caused by the DePuy Attune, it is anticipated that these lawsuits will be consolidated for discovery and other pretrial proceedings.
When cases are consolidated in this way in federal court it is called a multi-district litigation (MDL), and on a state level it is known as a state court consolidated proceeding. MDLs are distinct from class actions, and it is generally agreed that consolidating cases instead of proceeding in a class action is a more efficient and effective way of handling claims arising from injuries causes by medical devices.
Have There Been DePuy Attune Settlements?
Some cases settle early in the claims process, but it is not expected that there will be early DePuy Attune lawsuit settlements. In most cases that proceed in an MDL or state court consolidated proceedings, after a certain period of time initial trials, also known as bellwether trials, take place. The purpose of these trials is for the parties to get an idea of the types of evidence and arguments that will made, as well as to see how juries will respond to the evidence and arguments. After a certain number of cases have been tried, the parties are in a better position to determine whether a case can be settled.
It is expected that DePuy Attune lawsuit settlements will follow this pattern, although the outcome of any case is never guaranteed and past results are not necessarily predictive of future outcomes.
(888) 888-0612
DePuy Attune Lawsuit News
- March 2011The DePuy Synthes Total Knee System was approved for marketing under the FDA’s 510(k) approval process.
- June 2015A recall of the Tibial Articulation Surface Trial was initiated, and later terminated in November 2015.
- April 2017The National Joint Registry of England, Wales and Northern Ireland reported that between 2012 and 2016 there were 93 deaths and 46 revisions related to the with the ATTUNE knee replacement device.
How a DePuy Attune Lawsuit Attorney Can Help
Medical device makers have a duty to provide safe products. If there are risks of harm associated with their devices, they also must provide adequate warnings. If a device maker fails to fulfill this duty, it could be held liable in lawsuits for injuries that may result.
People injured by a defective DePuy Attune knee replacement, may be eligible to recover money for:
 Medical Expenses
Medical Expenses
 Lost Wages
Lost Wages
 Pain and Suffering
Pain and Suffering
The families of those killed may be eligible to recover money for funeral expenses and the pain that comes with losing a loved one.
The Time to Pursue a Claim is Limited. Contact Us Today.
If you or a loved one received a DePuy Attune knee replacement, or if you have questions about a possible DePuy Attune lawsuit, contact the Attorney Group for more information. We can answer your questions in a free and confidential consultation, and there is no obligation on your part to speak with us. If you have a case, we can connect you with an affiliated DePuy Attune lawsuit attorney who can assist you through the legal process. State laws limit the time you have to pursue a claim, so contact us today.





