Nexium Lawsuit
A Nexium lawsuit may be an option for patients who suffered complications after taking the heartburn medication for acid reflux disease. Complications such as chronic kidney disease, bone fractures, kidney failure, and hypomagnesemia have been linked to Nexium, one of the leading drugs on the market, and other proton pump inhibitors. Affected patients may be eligible to seek compensation with the help of a bad drug attorney.
For more information, contact Attorney Group today. Our consultations are free, confidential and without any obligation on your part. We can help answer your questions, and if you choose to pursue a claim we can connect you with an affiliated Nexium side effects attorney who can assist you throughout the legal process.

Have You Seen a Nexium Lawsuit Commercial?
 You may have seen a Nexium lawsuit commercial on television and wondered whether you or a loved one have been affected by Nexium and, if so, whether you are eligible to pursue a claim against the manufacturer or others. The purpose of this article is to provide you with additional information so that you have a better understanding of your options.
You may have seen a Nexium lawsuit commercial on television and wondered whether you or a loved one have been affected by Nexium and, if so, whether you are eligible to pursue a claim against the manufacturer or others. The purpose of this article is to provide you with additional information so that you have a better understanding of your options.
What Is Nexium?
The heartburn medication Nexium (esomeprazole magnesium) has recently been linked to chronic kidney disease, nephritis, renal failure and other serious side effects. Nexium is the brand name for esomeprazole, a drug used to treat certain stomach and esophagus problems. Nexium is approved for the treatment of gastroesophageal reflux disease (GERD), and in combination with antibiotics for the treatment of patients with H. pylori infection. It is also used to reduce the risk of gastric ulcers and in the treatment of Zollinger-Ellison syndrome.
Nexium relieves heartburn, difficulty swallowing, and persistent cough caused by acid reflux. It also helps heal acid damage to the stomach and esophagus, prevents ulcers, and may help prevent cancer in the esophagus.
Nexium belongs to a class of drugs called proton pump inhibitors (PPIs), which turn off the “acid pumps” in the stomach and block the production of acid.
Common Proton Pump Inhibitors (Brand Name and Generic Name):
- Dexilant (Dexlansoprazole)
- Nexium (Esomeprazole)
- Prevacid (Lansoprazole)
- Prilosec (Omeprazole)
- Protonix (Pantoprazole)
- AcipHex (Rabeprazole)
The Food and Drug Administration (FDA) approved esomeprazole in 2001. Pfizer bought the rights to the over-the-counter version of Nexium from AstraZeneca in 2012.
Nexium Side Effects
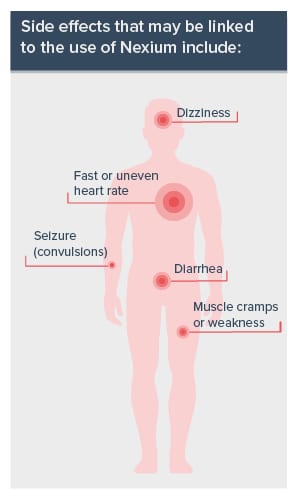 Side effects that may be linked to the use of Nexium include:
Side effects that may be linked to the use of Nexium include:
- Dizziness
- Fast or uneven heart rate
- Diarrhea
- Muscle cramps or weakness
- Seizure (convulsions)
Other Possible Nexium Complications include:
- Chronic Kidney Disease
- Kidney Failure
- Nephritis (kidney inflammation)
- Cardiac Disorders
- Allergic Reactions
- Dementia
- Hypomagnesemia (low magnesium levels)
- Bone fractures and broken bones
Nexium and Kidney Complications
| Lawsuits allege that, as a result of the defective nature of Nexium and other PPIs, people who use such medications may suffer from kidney injuries, including: | |
|---|---|
| Acute Interstitial Nephritis (AIN) – A type of kidney injury associated with an abrupt deterioration in renal function characterized by the inflammation of the renal interstitium, or the areas surrounding the tissues within the kidneys. Symptoms of AIN may include blood in the urine, fever, increased or decreased urine output, drowsiness, confusion, nausea, vomiting and weight gain. | |
| Acute Kidney Injuries (AKI) – Conditions in which the kidneys suddenly cannot filter waste from the blood; this usually occurs over a few hours or days. Symptoms include decreased urinary output, swelling due to fluid retention, nausea, fatigue and shortness of breath. AKIs can be fatal in some cases. | |
| Chronic Kidney Disease (CKD) – A kidney condition characterized by the slow, progressive loss of kidney function over a period of years. The condition eventually leads to permanent kidney failure and cannot be cured, however, there are treatment options available. | |
| Renal Failure or End-Stage Renal Disease (ESRD) – The last stage of chronic kidney disease when the kidneys no longer support the body’s needs. ESRD may be treated with dialysis or, in some cases, a kidney transplant may be needed. | |
Although Nexium is one of the most popular drugs on the market (between 2008 and 2013, over $50 billion worth of PPIs were sold), research has linked the use of PPIs to AIN, AKI, CKD and ESRD.

Lauren A. on May 16, 2016
Attorney Group reviewed by:"These guys are a pleasure to work with -- very strategic and very responsive, which makes for a great business partner! I can tell that they are passionate about making sure all clients get the attention and expertise they deserve."Rating: 5 ★★★★★
FDA Bone Fracture Warning
In May 2010, the FDA warned that there may be increased risk of bone fracture of the hip, wrist, and spine from prescription strength acid reflux drugs like Nexium. The FDA required an update to the warning label about the risk of fractures from Nexium and other drugs in the class.
FDA investigators determined that the risk of fractures is dose-specific and have not applied the Nexium bone fracture warning requirements to the over-the-counter versions of Nexium or similar PPIs. A specific risk of long-term use of Nexium is an increased risk of hip fractures in women with a history of smoking. A study published in the British Medical Journal found the associated risk with chronic use of PPIs.
Has There Been A Nexium Recall?
As of January 2017, there is no known recall of Nexium. However, in May 2010, the FDA announced that due to the strong association between PPIs and various types of bone fractures, the packaging on these type of drugs, including Nexium, would be required to carry an enhanced warning regarding the risks of these side effects. In 2014, the FDA also required the Nexium label to carry a warning about acute interstitial nephritis (a swelling in between the kidney tubules).
Although there has not been a Nexium recall, lawsuits claim that the drug makers failed to disclose known side effects of the drug and that patients suffered damages as a result. Failure to warn of side effects of a drug can be a basis of drug company liability, regardless of whether the drug has been recalled.
(888) 888-0612
Is There a Nexium Class Action?
There is no Nexium class action pending as of January 2017. Nexium lawsuit attorneys are doubtful that a class action will be certified for patients who are adversely affected by the drug. Instead, in light of recent studies relating to Nexium side effects, if multiple Nexium lawsuits are filed against the drug makers alleging injuries and other damages caused by Nexium and similar drugs, it is anticipated that these lawsuits will be consolidated for discovery and other pretrial proceedings.
When cases are consolidated in this way in federal court it is called multidistrict litigation (MDL), and on a state level it is known as a state court consolidated proceeding. MDLs are distinct from class actions, and it is generally agreed that consolidating cases instead of proceeding in a class action is a more efficient and effective way of handling claims arising from injuries caused by pharmaceutical products.
Have There Been Nexium Settlements?
Some cases settle early in the claims process, but it is not expected that there will be early Nexium settlements. In most cases that proceed in an MDL or state court consolidated proceedings, after a certain period of time initial trials, also known as bellwether trials, take place. The purpose of these trials is for the parties to get an idea of the types of evidence and arguments that will made, as well as to see how juries will respond to the evidence and arguments. After a certain number of cases have been tried, the parties are in a better position to determine whether a case can be settled.
It is expected that Nexium settlements will follow this pattern, although the outcome of any case is never guaranteed and past results are not necessarily predictive of future outcomes.
We can answer your questions in a free and confidential consultation.
Free Case ReviewCall Today: (888) 888-0612Nexium Lawsuit Claims
Lawsuits filed against AstraZeneca, manufacturer of Nexium, claim the drug maker engaged in conduct likely to mislead patients and consumers. Lawsuits allege that the manufacturer knew or should have known about the correlation between the use of Nexium and the increased risks of AIN, CKD, AKI and other renal impairments.
Despite knowledge of the alleged risks associated with Nexium, AstraZeneca continued to market and sell the medication without properly warning patients or health care providers of the significant kidney risks.
Some Nexium lawsuits claim up to 70 percent of PPIs may be prescribed and used inappropriately for indications or periods of time that were never tested or approved. Lawsuit further alleges that, as a result of the defective nature of PPIs people who take the drugs may have been exposed to significant risks, even if the medication was used as directed by a physician or health care professional.
(888) 888-0612
Nexium Lawsuit News
- January 2017Several Nexium lawsuits claim that the drug maker ignored the serious risks associated with the popular acid reflux medication, including the increased risk of kidney disease.
- November 2016Lawsuit filed alleges use of Nexium led to the plaintiff’s development of kidney injuries and that the drug maker attempted to conceal their knowledge of the dangers associated with the medication.
- February 2016A study suggests that taking Nexium and other proton pump inhibitors may increase a person’s risk for kidney failure and kidney disease.
- April 2015A study suggests that long-term use of Nexium and other PPIs may be linked to an increased risk of nephritis or kidney inflammation and injury.
- December 2014The FDA required the Nexium label to carry a warning about acute interstitial nephritis.
- March 2011The FDA announced that PPIs may be associated with low magnesium levels.
- May 2010The FDA requires Nexium and other PPIs packaging to carry an enhanced warning regarding bone fracture risks associated with the drug.
How a Nexium Lawsuit Can Help
Drug makers have a duty to provide safe products. If there are risks of harm associated with their products, they also must provide adequate warnings. If a drug maker fails to fulfill this duty, it could be held liable in lawsuits for injuries that may result.
People injured by Nexium may be eligible to recover money for:
 Medical Expenses
Medical Expenses
 Lost Wages
Lost Wages
 Pain and Suffering
Pain and Suffering
The families of those who have died may be eligible to recover money for funeral expenses and the pain that comes with losing a loved one.





