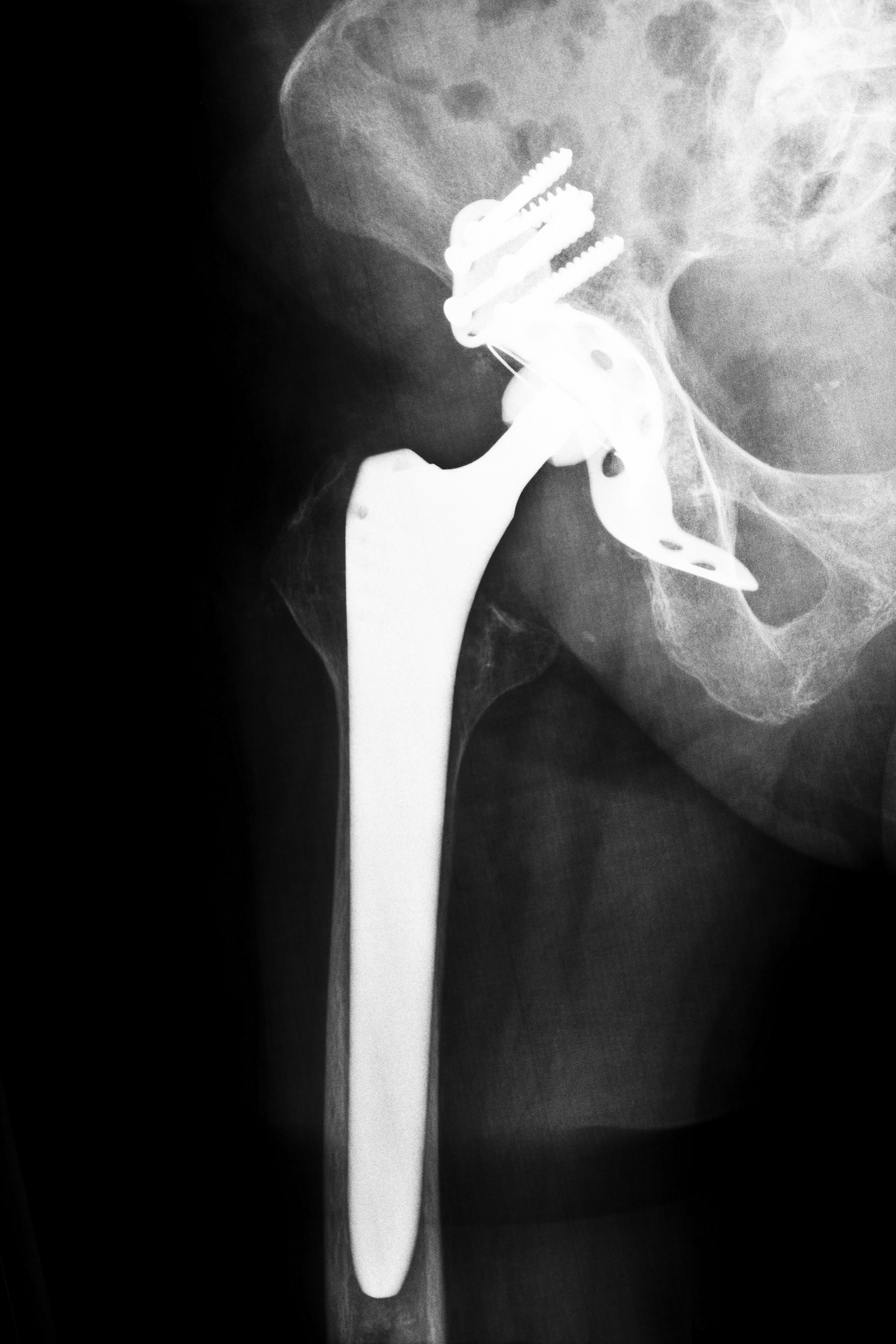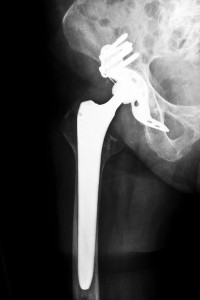
In the first of more than 10,000 U.S. lawsuits filed over now-recalled DePuy hip implants to go to trial, Johnson & Johnson has been ordered to pay over $8.3 million in compensatory damages after a Los Angeles jury found that the company defectively designed its DePuy ASR hip replacement systems. The jury found that the Johnson & Johnson defective hip implant device made by DePuy Orthopaedics, a division of Johnson & Johnson, caused injury to Loren Kransky, a 65 year old retired Montana prison guard.
Johnson & Johnson, the largest seller of health products in the world, recalled 93,000 DePuy ASR products in August, 2010, five years after the metal-on-metal hip replacement systems had been introduced into the U.S. market. The company cited higher than expected rates of premature device failures as the reason for pulling the devices from markets worldwide. The average hip implant is designed to last for 15 years or more, and rates of failures or dislocations in the first 5 years typically average between one and five percent. According to information brought forth during this trial, 12 percent of DePuy ASR devices failed within the first five years of implantation. In Australia, patient registration data showed a 44 percent failure rate within 7 years. Johnson & Johnson has argued that faulty surgical techniques have contributed to high failure rates, alleging that surgeons positioned the implants improperly, and have disputed the assertion that the design of DePuy ASR hip replacement systems is flawed.
The plaintiff in this case was fitted with DePuy’s ASR XL hip implant device in 2007 during a hip replacement procedure. In the years following that procedure, he suffered complications that finally led to revision surgery in 2012, which is a procedure done to remove and replace the hip implant. According to his attorneys, complications suffered by Kransky included dangerously high concentrations of metal ions in his blood, debilitating pain and difficulty walking, issues that caused him to be temporarily confined to a wheelchair. Kransky also stated that the constant pain caused by his Johnson & Johnson defective hip implant made sleep difficult, made it impossible for him to use the bathroom freely and caused him great difficulty as he attempted to rehabilitate after a stroke. Additionally, Kransky stated that the necessity for revision caused him extreme anxiety, as he was afraid that another surgery would result in his death.
Kransky’s attorneys argued that the blame for high rates of implant dislocations and revision surgeries lies with Johnson & Johnson defective hip implant design, and that the faulty design caused a variety of complications in patients besides implant dislocations and premature device failures. Debris shed from these metal-on-metal implants has been associated with high concentrations of metal ions in the bloodstream of patients, including Kransky, and some DePuy ASR recipients have suffered metallosis, a painful inflammatory reaction to implant debris that can cause serious injury to soft tissues and bones. They also argued that DePuy failed to test the device adequately before placing it on the market in 2005, and that the company buried reports made by surgeons on problems related to these hip replacement systems.
In defending the case, the company’s attorneys placed a great deal of emphasis on Kransky’s other health issues, including diabetes, cancer, heart disease, kidney disease and vascular disease, stating that they were unrelated to his hip and arguing that the Johnson & Johnson defective hip implant was not the cause of his injuries. Attorneys for Johnson & Johnson also argued that the case was not about the DePuy recall or the high revision rates associated with the device, but about the specific reasons Kransky’s ASR XL required replacement.
Kransky’s attorney sued for compensatory damages of $5.3 million and punitive damages of up to $179 million. After more than five days of deliberations, the jury delivered a verdict that included an award of $338,136 for Kransky’s medical expenses and $8 million in compensation for physical pain and emotional suffering caused by his experience with the Johnson & Johnson defective hip replacement device. While the panel ruled that the design of the metal-on-metal device was defective, it also found that DePuy properly warned of the risks, declining to award punitive damages in the case.
Through a spokesperson, Johnson & Johnson has stated that they feel that the DePuy ASR XL was properly designed and that actions taken by the company concerning the product were appropriate and responsible. Johnson & Johnson also announced that they intend to appeal the DePuy lawsuit verdict. The company will face the second of 10,750 lawsuits filed over the DePuy ASR beginning on March 11 in Chicago.