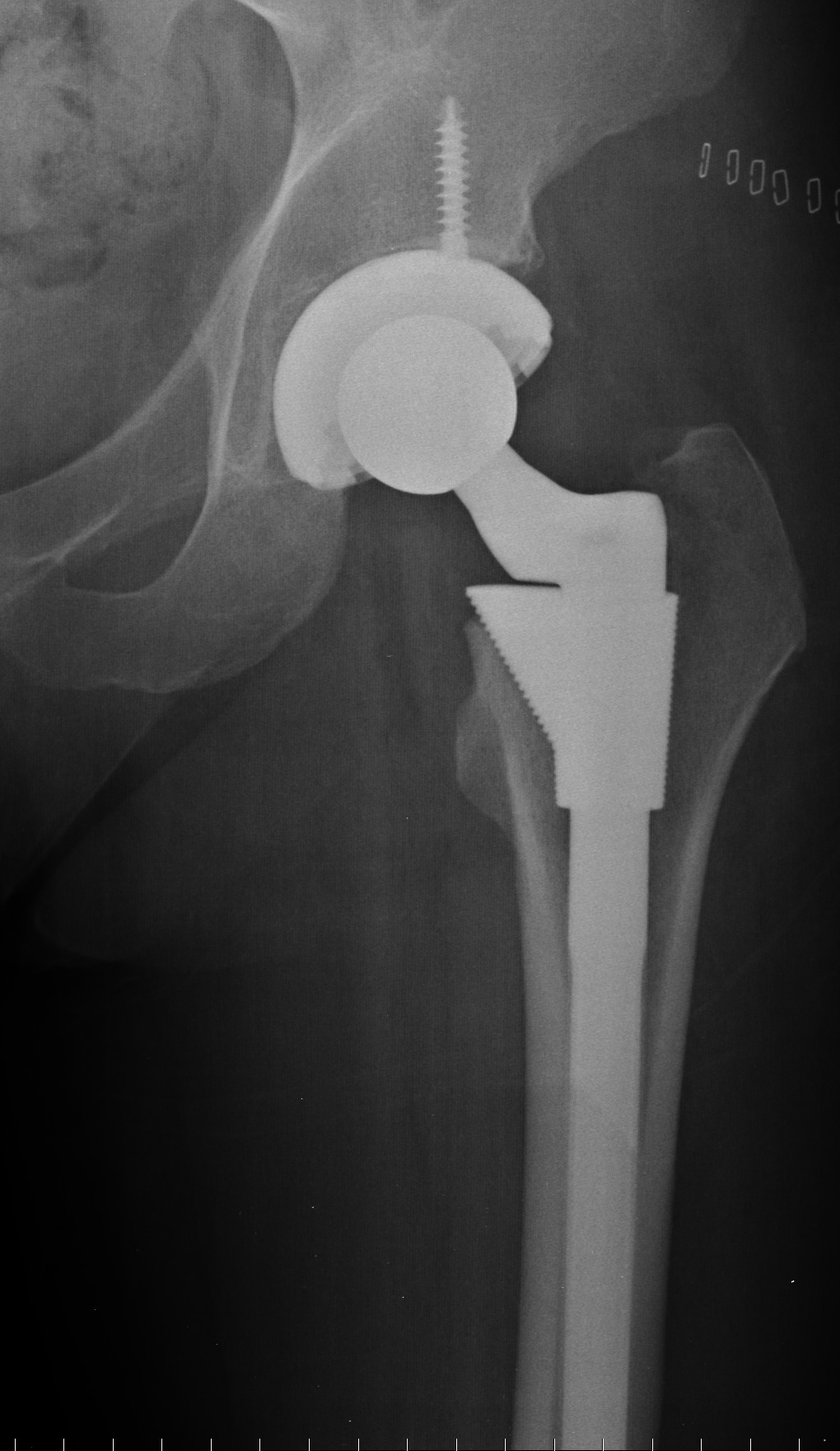
 Following the recall for the ASR hip replacement device in August 2010, DePuy ASR litigation is ongoing and in November 2014 defendant DePuy Orthopaedics and its parent company, Johnson & Johnson, announced a global settlement of approximately 8,000 lawsuits pending in state and federal courts throughout the U.S. While the settlement does not resolve all pending ASR hip replacement claims, and while patients might still be eligible participate in the DePuy ASR litigation and settlement, DePuy ASR recall attorneys are encouraging individuals who believe they have a claim to seek legal counsel as soon as possible.
Following the recall for the ASR hip replacement device in August 2010, DePuy ASR litigation is ongoing and in November 2014 defendant DePuy Orthopaedics and its parent company, Johnson & Johnson, announced a global settlement of approximately 8,000 lawsuits pending in state and federal courts throughout the U.S. While the settlement does not resolve all pending ASR hip replacement claims, and while patients might still be eligible participate in the DePuy ASR litigation and settlement, DePuy ASR recall attorneys are encouraging individuals who believe they have a claim to seek legal counsel as soon as possible.
If you would like more information on the DePuy ASR hip replacement settlement or to determine whether you are eligible to pursue your own claim, contact Attorney Group. We offer free, no-obligation consultations and can assist you in determining whether you have a claim.
DePuy Hip Replacement Recall and Lawsuits
Johnson & Johnson issued a global recall in August 2010 to advise 93,000 ASR implant recipients of possible complications with the device, reportedly due to studies showing that 12 percent of these devices had failed within the first five years following implantation. However, according to internal documents produced during litigation, 37 percent of ASR hips failed in as little as 4.6 years. In Australia in 2012, the reported failure rate was 44 percent within seven years.
The company likewise ceased distribution of its Pinnacle hip in August 2013 after the U.S. Food and Drug Administration required all metal-on-metal hip implant manufacturers to submit new versions of their products for pre-market approval. However, Johnson & Johnson is not the only manufacturer facing metal-on-metal hip replacement litigation. Other companies (and their devices) include:
- Zimmer (Duron Cup)
- Stryker (ABG II and Rejuvenate Modular Neck Hip Stems)
- Smith & Nephew (R3 Acetabular System)
- Wright Conserve (Conserve line of replacement devices)
ASR Settlement Program Created
According to the settlement details, patients must have been fitted with a DePuy ASR hip implant and undergone a removal, or revision, procedure prior to August 31, 2014. Many patients underwent revision procedures before that date and are eligible to settle their product liability and personal injury claims against the manufacturers under the terms of the program.
Those who had revision surgeries after the cut-off date, or that believe that they should receive compensation in excess of the $250,000 base settlement award under the terms of the program. If you are experiencing complications with your DePuy ASR hip replacement, contact Attorney Group today for information on how you can still pursue a claim against the device maker even if you are not eligible to participate in the settlement program..
DePuy and Johnson & Johnson have reportedly set aside $2.5 billion to fund its settlement program. The medical device manufacturers have also been named as defendants in similar cases in which plaintiffs assert the company’s Pinnacle line of hip replacement devices are prone to metallosis, or metal poisoning, and premature device failure.
Have Questions about the DePuy ASR Hip Implant Settlement?
If you would like to learn more about filing a DePuy ASR hip implant lawsuit or participating in the DePuy ASR settlement program, contact Attorney Group for a free case evaluation. We can provide you with a comprehensive review of your case at no out-of-pocket cost to you, and if you are eligible to seek compensation for your injuries, we can refer you to an affiliated attorneys who can file a lawsuit on your behalf. You may be eligible to recover damages for pain and suffering, medical expenses, and lost wages.