In a court document filed on February 20, 2015, the committee overseeing the global settlement of DePuy metal on metal hip implant cases reached in November 2013 announced “an agreement which would effectively extend the existing U.S. Settlement Program to U.S. citizens/residents with ASRTM hips, who had revision surgery on or before January 31, 2015, subject to the terms and conditions of the Program.” Under the original terms of the DePuy ASR settlement, only patients who had revision surgery prior to August 31,2013, would be eligible to participate.
If you received a metal on metal hip implant before January 31, 2015 and would like to know more about the DePuy ASR settlement, contact Attorney Group. We provide free, no-obligation consultations and, if you have a case, we can connect you with a DePuy ASR hip replacement attorney who can assist you in pursuing your claim.
 DePuy Hip Implant Problems and Lawsuits
DePuy Hip Implant Problems and Lawsuits
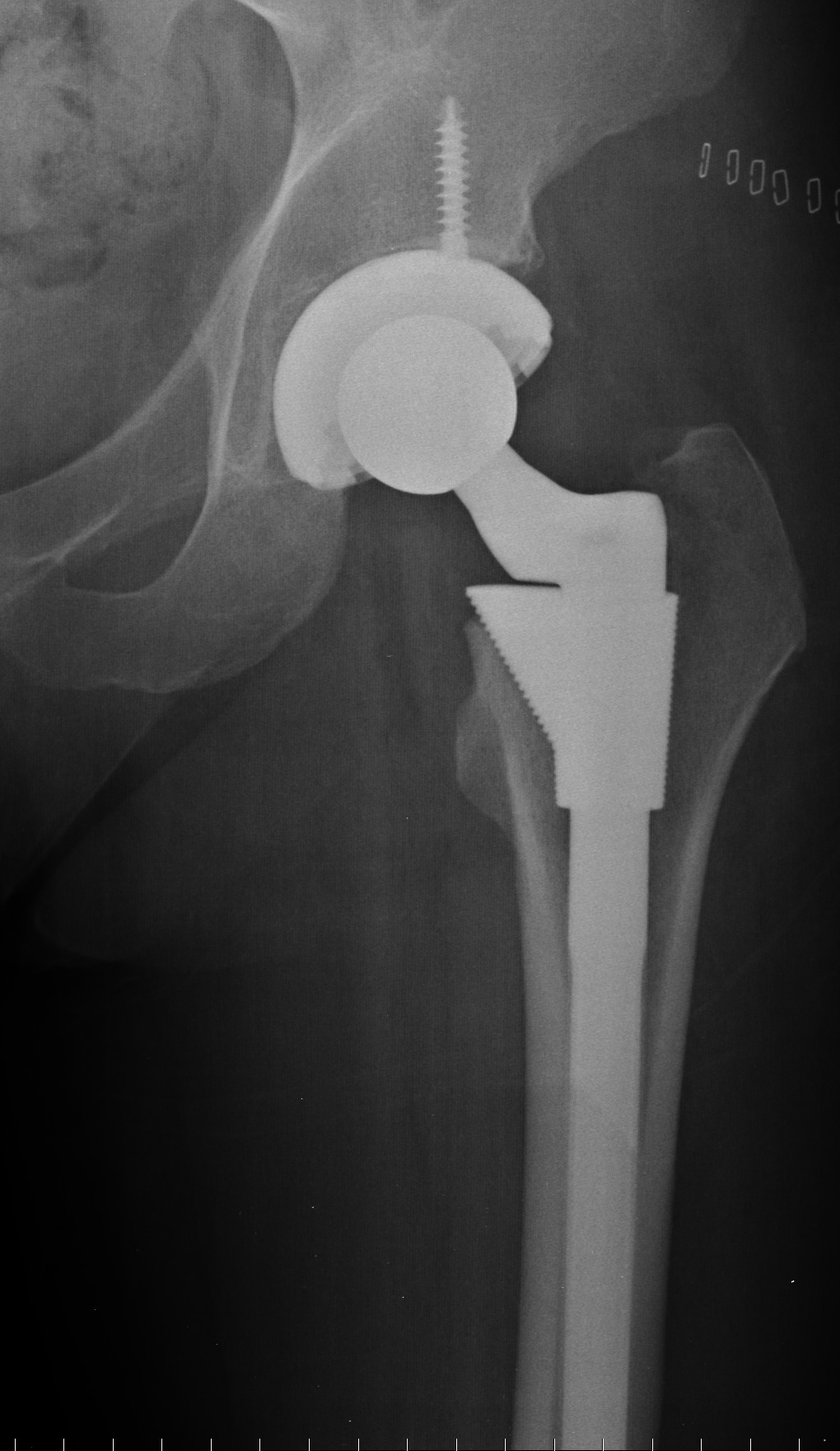
DePuy ASR hip systems were first introduced in 2005 and are among a class of metal on metal hip replacement systems that were intended to have higher durability than other types of hip implants, which typically combined a ceramic or metal ball with a plastic socket. Many device makers hoped that a more durable system would expand the market for hip replacements from traditionally older patients to younger, more active individuals.
After their introduction, however, metal on metal hip replacement patients, including those who received the DePuy ASR, began to experience higher than expected failure rates, and many had to undergo costly and painful revisions procedures where the original hip replacement had to be removed and replaced with a different type. Additionally, as the hip systems were found to shed metallic particles into the bloodstream and tissue surrounding the hip, many patients began experiencing symptoms associated with metallosis, including pain, the development of tumors, and neurological problems, which forced the revision of additional hip replacements.
Because of these and other problems, DePuy recalled its ASR hip replacements in 2010, and thousands of DePuy ASR hip replacement patients began filing lawsuits claiming that the devices were defective and that DePuy and it’s parent company, Johnson & Johnson, were negligent in the design of the product and intentionally withheld data showing problems with the device.
Settlement of Claims Reached in November 2013
In an effort to resolve approximately 7,500 of these claims, DePuy announced a settlement in November 2013 that would provide patients who had a revision of their DePuy ASR before August 31, 2013 a base award of $250,000, which could be increased or decreased depending on certain factors. The February 20, 2015 announcement extended eligibility to patients who had revisions prior to January 31, 2015, subject to the other terms and conditions of the settlement.
DePuy hip replacement attorneys note that the settlement is not binding on patients who choose to opt out of the program and pursue higher damages based on their particular case. However, as time is of the essence both in participating in the DePuy settlement program and in pursuing a claim outside of the settlement program, DePuy hip implant patients are encouraged to promptly seek legal counsel in order to discuss their options.
Have Questions About the DePuy ASR Settlement? Call Us.
If you have questions about the DePuy ASR settlement or would like more information about pursuing a claim for compensation based on your metal on metal hip replacement, contact Attorney Group today. We offer free, no obligation consultations and can help you explore your options and determine if you have a claim. We can also connect you with a metal on metal hip implant attorney who can assist you in pursuing a claim to recover medical expenses, lost wages, pain and suffering, and more.