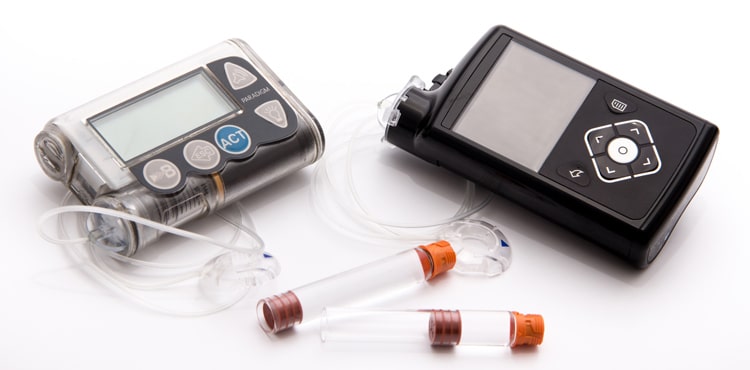
On February 12, the Food and Drug Administration (FDA) announced that Medtronic has issued a recall on certain models of its MiniMed 600 Series Insulin Pumps. The recalled insulin pumps may have “a missing or broken retainer ring which helps to lock the insulin cartridge into place in the pump’s reservoir compartment.” According to the FDA, if the cartridge is not locked firmly into place, an incorrect amount of insulin may be delivered, which could result in hypoglycemia or hyperglycemia.
If you or your loved one has used a Medtronic MiniMed Insulin Pump to treat diabetes and suffered injury as a result, you may be eligible to seek compensation with the help of a defective medical device lawsuit attorney.
For more information, contact Attorney Group. We offer free, confidential, no obligation consultations. We can help answer your questions, and if you choose to pursue a claim, we can connect you with an affiliated attorney who can assist you throughout the legal process.
FDA: Recalled Insulin Pumps Linked to One Death and Thousands of Injuries
People who have type 1 diabetes often use the MiniMed insulin pump to deliver insulin for the management of their diabetes. At the time of the announcement, Medtronic had received 26,421 complaints, and the company is aware of 2,175 injuries and one death linked to the potentially defective insulin pumps. Over 300,000 Medtronic MiniMed Insulin Pumps have been recalled in the United States, according to the FDA’s announcement.
Type 1 diabetes typically affects children, adolescents, and young adults; however, the condition can develop at any age. Model 630G insulin pumps are used by persons sixteen years of age and older, and model 670G insulin pumps are typically used by people fourteen years of age and older.
The following models have been included in the recall:
| Product (MiniMed 600 Series Insulin Pumps) | Distribution Dates |
| Model 630G (MMT-1715) – all lots before October 2019 | September 2016 to October 2019 |
| Model 670G (MMT-1780) – all lots before August 2019 | June 2017 to August 2019 |
On November 21, 2019 Medtronic notified affected customers and advised them to:
- examine the retainer ring of their pump;
- stop use of the pump and contact Medtronic for a replacement pump if the reservoir does not lock into the pump or if the retainer ring is loose, damaged, or missing;
- continue using the pump if the reservoir locks in place correctly;
- check the pump and retainer ring for damage and stop use if it is damaged; and
- check the pump retainer ring and verify that the reservoir is locked correctly at every set change.
If you stop using the pump, you should follow your doctor’s recommendations and perform manual insulin injections. The FDA has identified this as a Class I recall, the most serious type of recall. Use of these devices may cause serious injuries or death.
How Can an Attorney Help?
Medical device makers have a duty to provide safe products. If there are risks of harm associated with their devices, they are required to provide adequate warnings. If a device maker or manufacturer fails to fulfill this duty, it could be held liable in lawsuits for injuries that may result.
People injured by a Medtronic MiniMed Insulin Pump may be eligible to recover money for:
- Medical expenses
- Lost wages
- Pain and suffering
The families of those killed may be eligible to recover money for funeral expenses and the pain that comes with losing a loved one.
The Time You Have to Pursue a Claim is Limited. Contact Us Today.
For more information, contact Attorney Group. After you contact us, an attorney will follow up to answer questions that you might have.
There is no cost or obligation to speak with us, and any information you provide will be kept confidential.
Please note that the law limits the time you have to pursue a claim or file a lawsuit for an injury. If you think you have a case, you should not delay taking action.