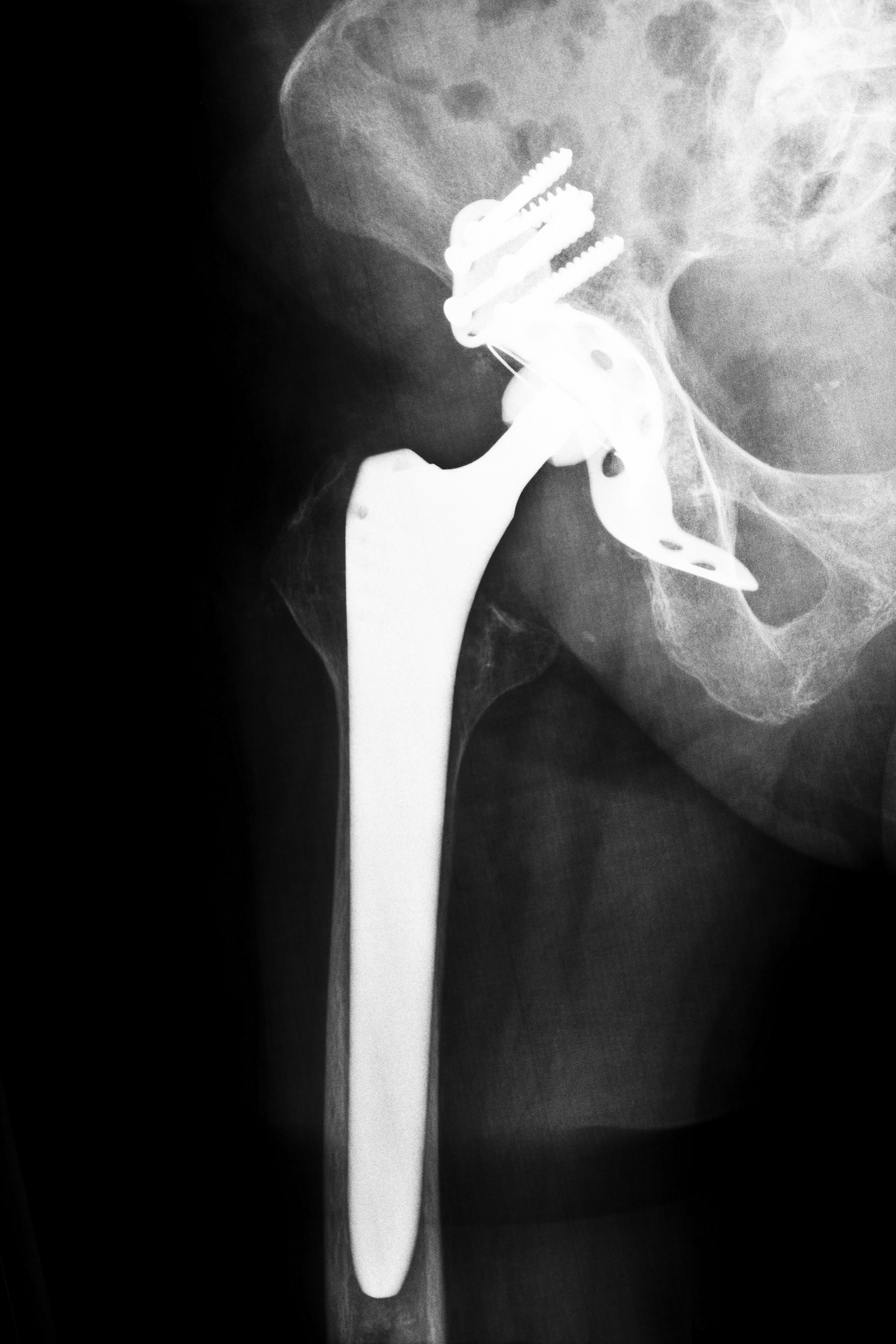
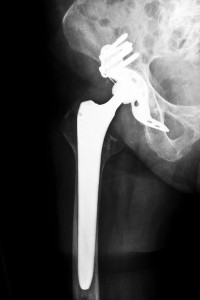
 An important milestone in the DePuy hip recall was reached after DePuy Orthopaedics announced it was waiving its right to walk away from a settlement agreement. The announcement came several weeks before a court-imposed deadline required their decision. Their agreement to waive the right to walk away means the DePuy hip recall legal action can move into enacting the Settlement Program to pay the affected patients who chose to participate in the program. The exact amount of compensation provided is specific to each case.
An important milestone in the DePuy hip recall was reached after DePuy Orthopaedics announced it was waiving its right to walk away from a settlement agreement. The announcement came several weeks before a court-imposed deadline required their decision. Their agreement to waive the right to walk away means the DePuy hip recall legal action can move into enacting the Settlement Program to pay the affected patients who chose to participate in the program. The exact amount of compensation provided is specific to each case.
The decision to waive was announced by the U.S. District Court for the Northern District of Ohio on May 20th, 2014. Johnson & Johnson, the parent company of DePuy Orthopaedics, agreed to pay $2.5 billion out to settle some 8000 lawsuits regarding the DePuy hip recall. The legal action involved the ASR XL and Resurfacing hip implant and will continue to move forward to resolve the cases disallowed from the settlement agreement.
Depuy Hip Recall Settlement
The DePuy hip recall settlement waiver affects just one multidistrict litigation (MDL). There are still thousands of lawsuits that need to be resolved in various states depending on the type of implant.
The Texas court hearing the Pinnacle hip recall lawsuits has received a request to allow live transmission testimony from remote witnesses maintained by the defense. The supporting arguments from plaintiffs include the fact that those out of state did not have a choice in where their DePuy hip recall lawsuit was held and that a videoconference will not have an unfair, negative affect on the defense. Courts prefer live testimony, so videoconferencing seems to be the most viable solution for the additional testimony.
The Source Of Legal Action
DePuy hip recalls are largely at the source of this body of lawsuits, particularly metal on metal hip implants. Plaintiffs allege that DePuy did not do enough testing before pushing their product into the public market. Erosion of the devices from their metal on metal movement sheds metal ions into the body. The result is problems like metal poisoning, chronic pain, pseudotumor development, and premature device failure.
Several devices have also been subject to revision surgeries. A revision surgery requires the surgeon remove the original implant and put in a new one soon after the installation. These implants are meant to last for years, but recalls and damage have created the need for revisions in many patients within a year or two of receiving an implant.
Legal Consultation
Legal action surrounding the DePuy hip recall is based on products that have proven to be harmful to the patients they are installed in. Patients should not need to suffer more due to the alleged negligence of manufacturers. It is important to be informed about legal options if you or a loved one has been negatively affected by the DePuy hip recall. The fact that these MDLs are moving forward means that deadlines could apply for patients that want to seek damages.
If you or a loved one have dealt with pain or injury after receiving a hip implant involved in the Depuy hip recall, contact Attorney Group today. We can help answer questions, help you determine if you have a claim, and connect you with an affiliated attorney who can help you through the legal process. Contact us today for a free consultation.