What is Abilify?
Abilify (aripiprazionle) is a medication used to treat the symptoms of psychotic conditions such as depression, autism and bipolar disorder. It works by changing the actions of chemicals in the brain. This antipsychotic is mostly prescribed to treat the symptoms of schizophrenia, a mental illness that causes disturbed or unusual thinking, loss of interest in life, and strong or inappropriate emotions, in adults and teenagers 13 years of age and older.
It can also be used alone or with other medications to treat episodes of mania and other abnormal moods in subjects affected by manic-depressive disorder, a disease that causes episodes of depression, episodes of mania, and other abnormal moods.
First introduced in the U.S. in 2002, belongs to a class of medications called atypical antipsychotics. It works by normalizing the activity of dopamine and serotonin in the central nervous system (CNS).

Abilify and Compulsive Behaviors
The antipsychotic medication Abilify has recently been linked to compulsive behaviors including gambling, shopping, hypersexuality, and loss of impulse control. These serious side effects have resulted in substantial financial, mental and physical damages to patients who have taken the drug.
Compulsive gambling is a major psychiatric disorder, first recognized in the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1980. Numerous clinical studies reported a strong association between the use of this drug and the development of serious form of loss of impulse control.
Plaintiffs claim that drug makers Bristol-Myers Squibb and Otsuka Pharmaceutical knew, or should have known, that the drug could lead to an increased risk of uncontrollable compulsive behaviors. However, they willingly failed to report these risks in the United States, as the label for the medication in Europe and Canada was already required to include such warnings.
On May 3, 2016, the U.S. Food and Drug Administration (FDA) released a safety communication to alert the public about reported instances of compulsive urges, with the use of Abilify. The U.S. label for the drug did not contain any mention of these issues prior to January 2016, however.
Why You Should File an Abilify Lawsuit?
Filing a litigation may be an option to recover compensation for patients who took the medication and suffered from the unwanted adverse reactions associated with this drug. Plaintiffs claim that drug makers Bristol-Myers Squibb and Otsuka Pharmaceutical knew, or should have known, that the drug could lead to an increased risk of serious and dangerous side effects, including uncontrollable compulsive behaviors and loss of impulse control.
However, claims allege that the manufacturers voluntarily hide these risks to increase their profits and willfully failed to warn. Abilify was Bristol-Myers’ largest-selling product in 2013, with sales of $2.3 billion, and worldwide revenues of $555 million over three months ending June 30, 2014.
If you suffered from compulsive gambling, shopping, binge eating or irresistible urges that resulted in financial loss while taking Abilify, or if you want more information your legal options, contact Attorney Group today. Our consultations are free, confidential and without any obligation on your part.
We can answer your questions in a free and confidential consultation.
Free Case ReviewCall Today: (888) 888-0612Other Abilify Risks
In addition to the alleged relationship between the use of Abilify and compulsive behaviors, there are a number of other risks associated with the prescription antipsychotic. According to the NIH’s drug information, studies reportedly show that older adults with dementia who take antipsychotics, including Abilify, have an increased chance of death during treatment.
Older adults with dementia may also have a greater chance of having a stroke or ministroke or other severe side effects during treatment. It is also noted that the FDA has not approved Aripiprazole for the treatment of behavior problems in older adults with dementia.
People who have depression and take antipsychotics are especially warned that a small number of children, teenagers, and young adults (up to 24 years of age) who took medications for depression during clinical studies became suicidal. Children, teenagers, and young adults who take antidepressants to treat depression or other mental illnesses may be more likely to become suicidal than children, teenagers, and young adults who do not take antidepressants to treat these conditions.
Abilify Side Effects
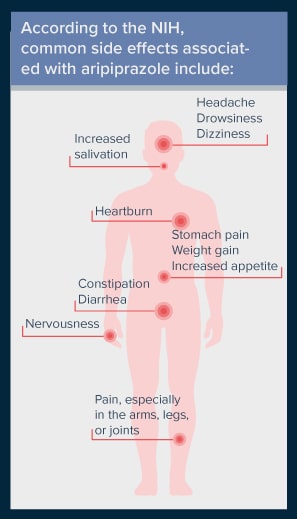
According to the NIH, common side effects associated with aripiprazole include:
- Headache
- Nervousness
- Drowsiness
- Dizziness
- Heartburn
- Constipation
- Diarrhea
- Stomach pain
- Weight gain
- Increased appetite
- Increased salivation
- Pain, especially in the arms, legs, or joints
Other side effects, including chest pain, high fever and changes in vision, are noted as serious and patients are advised to report them to their doctor immediately.
Has There Been an Abilify Recall?
Although the FDA has issued a Safety Alert to warn patients about its potential risks, there has not been an Abilify recall, yet.
Although many lawyers claim that the drug makers failed to disclose known side effects of the medication which severely harmed patients as a result, none of these adverse reactions forced the pharmaceutical companies to withdraw it from the market.
However, failure to warn of side effects of a drug can be a basis of a defective product liability, regardless of whether the medicine has been recalled or not.
Is the Abilify Litigation a Class Action or a Mass Tort Action?
There is no Abilify class action pending as of December, 2017. To date, most attorneys are doubtful that this type of litigation will be certified for patients who are adversely affected by the drug.
Instead, since multiple lawsuits have been filed against the manufacturers alleging similar injuries and other damages caused by this products, they have been centralized in a multidistrict litigation (MDL).
The purpose of an MDL is to speed up the discovery phase and other pretrial proceedings. Cases are consolidated in this way in federal court since it is a more efficient and effective way of handling claims, and it provides plaintiffs with a greater chance to receive a substantial financial compensation.
Currently, almost 400 suits have been centralized in the MDL No. 2734 in the U.S. District Court for the Northern District of Florida.
"These guys are a pleasure to work with -- very strategic and very responsive, which makes for a great business partner! I can tell that they are passionate about making sure all clients get the attention and expertise they deserve."Attorney Group Reviewed by Lauren A. on .
Has There Been an Abilify Settlement?
On December 8, 2016, the New York State Office of the Attorney General announced a $19.5 million agreement with the manufacturer of Abilify, Bristol-Myers Squibb, resulting from allegations surrounding inappropriate marketing and promotion of the antipsychotic medication.
According to a statement issued by New York Attorney General Eric T. Schneiderman, alleged off-label marketing practices and misleading claims about the drug sparked the complaint filed in New York County Supreme Court. Forty-one states and the District of Columbia participated in the settlement, which included a number of injunctive terms prohibiting the manufacturer from promoting this drug for off-label use.
The History of the Abilify Legal Proceedings
- December 2016New York State Attorney General announces a $19.5 million agreement between Bristol-Myers Squibb and 41 states and the District of Columbia regarding allegations of improper marketing and promotion of Abilify.
- October 2016According to a transfer order issued in early October 2016, the U.S. Judicial Panel on Multidistrict Litigation (JPML) has decided to centralize over 20 product liability lawsuits to the U.S. District Court for the Northern District of Florida.
- May 2016The FDA issues a safety alert stating that “Although pathological gambling is listed as a reported side effect in the current aripiprazole drug labels, this description does not entirely reflect the nature of the impulse-control risk FDA identified.”
- 2015Canadian regulators concluded that there is “a link between the use of aripiprazole and a possible risk of pathological gambling or hypersexuality” linked with the use of this medication.
- 2012The European Medicines Agency (EMA) requires that drug makers warn patients and the medical community in Europe that Abilify use included the risk of pathological gambling.
Have You Seen an Abilify Lawsuit Commercial?
You may have seen an Abilify lawsuit commercial on television and wondered whether you or a loved one have the right to file a case in court. If you have been negatively affected by the undisclosed effects of this drug, you might have lost thousands of dollars in gambling, shopping or medical bills.
If so, choosing the best lawyer available to pursue a claim against the manufacturer is critical. Don’t just call the first phone number you saw advertised on TV.
We at Attorney Group will provide you a free legal consultation, and will advise you on which is the most competent law firm you might want to call to assist you throughout the legal process.
How Filing a Litigation Can Help You
How Filing a Litigation Can Help You
When patients suffer from severe side effects from a dangerous or defective drug, they may be entitled to compensation for their injuries and damages.
The types of losses that can be recovered include:
Medical and other treatment expenses
Economic loss
Mental anguish
If the conduct of a drug company in manufacturing and selling drug is found to be highly reckless, punitive damages may be awarded to punish them and deter similar conduct in the future.
If you suffered any such loss as a consequence of being prescribed with a defective drug such as Abilify, we encourage you to pursue a claim as soon as possible.
Call us now, we’re here to help you.




